What Does Aq Mean In A Chemical Equation
Juapaving
Mar 17, 2025 · 5 min read
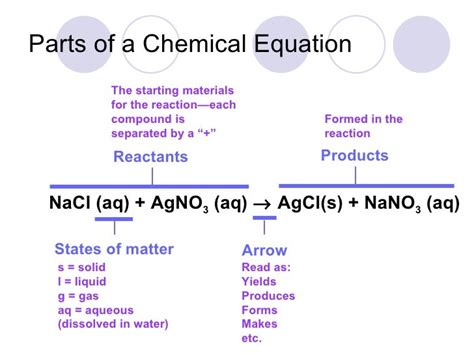
Table of Contents
What Does Aq Mean in a Chemical Equation? A Comprehensive Guide
In the world of chemistry, understanding the symbols and notations used in chemical equations is crucial for interpreting reactions and predicting outcomes. One such symbol that often arises is "aq," a shorthand notation that plays a significant role in describing the state of reactants and products. This comprehensive guide delves deep into the meaning of "aq" in chemical equations, exploring its implications, usage, and significance in various chemical contexts.
Decoding "aq": The Aqueous State
In chemical equations, "aq" is an abbreviation for aqueous, which signifies that a substance is dissolved in water. Water, being a highly polar solvent, effectively dissolves many ionic compounds and polar molecules, leading to the formation of homogeneous solutions. When a substance is designated as (aq), it indicates that the substance exists as individual ions or molecules dispersed uniformly throughout the water solvent, rather than in a solid, liquid, or gaseous phase.
This seemingly simple notation carries significant weight, influencing how we understand and represent chemical processes. It highlights the crucial role of the solvent in chemical reactions, shaping reaction pathways, influencing reaction rates, and impacting the overall outcome.
Significance of the Aqueous State in Chemical Reactions
The designation (aq) isn't merely a label; it profoundly impacts our understanding of chemical reactions in several ways:
-
Ionization: Many ionic compounds, when dissolved in water, dissociate into their constituent ions. For instance, sodium chloride (NaCl), a common table salt, dissolves in water to yield sodium ions (Na⁺) and chloride ions (Cl⁻). This process is represented as:
NaCl(s) → Na⁺(aq) + Cl⁻(aq)
The (aq) notation clearly indicates that the ions are now dispersed in the aqueous solution.
-
Solubility: The (aq) notation reflects the solubility of a substance in water. If a substance is insoluble in water, it will be represented by its physical state (s for solid, l for liquid, or g for gas). For example, silver chloride (AgCl) is insoluble in water and is represented as AgCl(s) in chemical equations.
-
Reaction Mechanisms: The state of reactants greatly affects the mechanism of a chemical reaction. Reactions involving aqueous species often proceed through different pathways than reactions involving solids or gases. For example, reactions in aqueous solutions may involve ion-dipole interactions or collision between dissolved ions, whereas reactions involving solids might necessitate surface interactions.
-
Reaction Rates: The concentration of reactants in the aqueous phase significantly impacts the rate of reaction. The higher the concentration of dissolved reactants, the higher the likelihood of collisions and the faster the reaction rate, all other factors being equal.
-
Equilibrium: The (aq) notation is particularly important when considering equilibrium reactions. The concentration of aqueous species directly influences the position of equilibrium, as described by the equilibrium constant (K).
Contrasting (aq) with Other State Symbols
Understanding the implications of "aq" requires comparing it to other state symbols commonly used in chemical equations:
-
(s) – Solid: Indicates that the substance exists as a solid. The particles are closely packed, with strong intermolecular forces holding them together. Reactions involving solids often occur at the surface of the solid.
-
(l) – Liquid: Represents the substance in its liquid state. Particles are more mobile than in solids but still experience significant intermolecular forces. Liquid-phase reactions involve interactions and collisions between molecules.
-
(g) – Gas: Denotes that the substance is in the gaseous phase. Particles are widely dispersed, with weak intermolecular forces. Gas-phase reactions involve frequent collisions between molecules and are often sensitive to pressure and temperature changes.
-
(l) vs. (aq): While both represent liquids, (aq) specifically means dissolved in water. A pure liquid, like ethanol, is represented as (l). However, if the ethanol is dissolved in water, it would be denoted as (aq).
Examples of (aq) in Chemical Equations
Let's examine several examples to illustrate the usage of (aq) in diverse chemical contexts:
1. Acid-Base Neutralization:
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Here, all the reactants and products, except water, are in the aqueous state. The reaction involves the neutralization of H⁺ ions from the acid and OH⁻ ions from the base.
2. Precipitation Reactions:
Precipitation reactions involve the formation of an insoluble solid (precipitate) from a mixture of soluble ionic compounds. For example, the reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl):
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
Note that silver chloride (AgCl) is insoluble and is therefore represented as (s), whereas other substances are in the aqueous state.
3. Redox Reactions:
Redox reactions involve the transfer of electrons. Many redox reactions occur in aqueous solutions. For instance, the reaction between iron(II) ions and potassium permanganate:
5Fe²⁺(aq) + MnO₄⁻(aq) + 8H⁺(aq) → 5Fe³⁺(aq) + Mn²⁺(aq) + 4H₂O(l)
This equation shows the oxidation of Fe²⁺ and reduction of MnO₄⁻ in an acidic aqueous solution.
4. Complex Ion Formation:
Complex ions are formed when a central metal ion is surrounded by ligands (molecules or ions). Many complex ion formation reactions occur in aqueous solutions:
Cu²⁺(aq) + 4NH₃(aq) → [Cu(NH₃)₄]²⁺(aq)
This shows the formation of a tetraamminecopper(II) complex ion in an aqueous solution.
Advanced Considerations: Activity and Concentration
While (aq) indicates a substance is dissolved in water, it simplifies the reality of the solution. In more rigorous thermodynamic calculations, the concept of activity replaces concentration. Activity accounts for the non-ideal behavior of ions in solution, such as interionic attractions that can affect the effective concentration.
Concentration is another important aspect. The (aq) notation doesn't specify the concentration. The concentration of aqueous species can vary widely, influencing reaction rates and equilibrium positions. Therefore, in practical applications, it's crucial to know or determine the concentration of the aqueous species involved.
Conclusion: The Importance of Precise Notation
The seemingly simple (aq) notation in chemical equations represents a pivotal aspect of chemical reactions. It highlights the critical role of the solvent, influencing reaction mechanisms, rates, and equilibrium. By understanding its implications and comparing it to other state symbols, chemists gain a more precise and accurate representation of chemical processes. Furthermore, appreciating the nuances of activity and concentration provides a more comprehensive perspective on the behavior of substances in aqueous solutions. The correct and consistent use of (aq) is essential for precise communication and accurate interpretation of chemical reactions. Mastering this seemingly minor detail forms a cornerstone of chemical understanding and enhances the ability to navigate the complexities of chemical processes.
Latest Posts
Latest Posts
-
Least Common Multiple Of 6 9 And 12
Mar 17, 2025
-
Write 80 As A Product Of Prime Factors
Mar 17, 2025
-
Which Is A Better Conductor Of Electricity Metal Or Water
Mar 17, 2025
-
How Many Minutes Are There In A Day
Mar 17, 2025
-
Surface Area And Volume Class 10
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Does Aq Mean In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
