What Are Three Examples Of A Chemical Change
Juapaving
Mar 31, 2025 · 7 min read
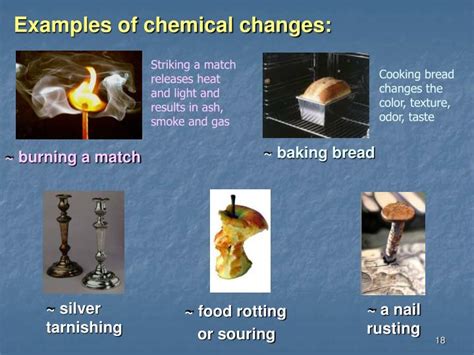
Table of Contents
What Are Three Examples of a Chemical Change? A Deep Dive into Chemical Reactions
Understanding the difference between physical and chemical changes is fundamental to grasping the basics of chemistry. While a physical change alters the form or appearance of a substance without changing its chemical composition (like melting ice), a chemical change, also known as a chemical reaction, results in the formation of new substances with different properties. This article will explore three compelling examples of chemical changes, delving into the processes involved, the observable changes, and the underlying chemical principles.
1. Burning a Candle: Combustion and Oxidation
One of the most readily observable examples of a chemical change is the burning of a candle. This seemingly simple process is actually a complex chemical reaction involving combustion, a rapid reaction between a substance and an oxidant, usually oxygen, that produces heat and light. Let's break down what happens:
The Chemistry of Candle Burning
A candle is primarily composed of wax, a hydrocarbon, meaning it's made up of carbon and hydrogen atoms bonded together. When you light a candle, the heat from the flame melts the wax near the wick. This liquid wax is then drawn up the wick via capillary action. In the flame's intense heat, the wax vaporizes. Oxygen from the surrounding air comes into contact with the hot wax vapor. This interaction initiates a combustion reaction.
The overall reaction can be simplified (though it's much more complex in reality) as follows:
Hydrocarbon (Wax) + Oxygen → Carbon Dioxide + Water + Heat + Light
Observable Changes: Evidence of a Chemical Change
Several observable changes confirm that burning a candle is a chemical change:
- Production of Light and Heat: The most obvious signs are the flame itself, emitting both light and significant heat. This energy release is a hallmark of exothermic reactions, where energy is released to the surroundings.
- Formation of New Substances: The original wax is transformed into carbon dioxide (a gas) and water (a vapor initially, then condensing into liquid), neither of which possesses the same properties as the original wax.
- Irreversibility: You cannot easily reverse the process. Once the wax has burned, you can't simply put the carbon dioxide and water back together to get the original wax. This irreversibility is another key characteristic of a chemical change.
- Change in Mass (Slightly): While the overall mass might seem to decrease (as the wax burns away), a careful measurement would show a slight increase in mass due to the oxygen that reacts with the wax. This is often overlooked but demonstrates the involvement of another reactant.
- Production of Soot (Carbon): In an incomplete combustion reaction, some carbon particles (soot) may also be produced, indicating that not all the wax was completely oxidized to carbon dioxide. This soot is a further indication of a chemical transformation.
Deeper Understanding: The Role of Oxygen
The presence of oxygen is crucial for combustion. If you were to cover a burning candle with a jar, the flame would eventually extinguish. This is because the oxygen supply within the jar becomes depleted, preventing further oxidation of the wax. This demonstrates the vital role of an oxidant in combustion reactions.
2. Rusting of Iron: Oxidation and Corrosion
Rusting, the formation of iron oxide, is another classic example of a chemical change. It's a process of oxidation, where iron reacts with oxygen in the presence of water (or moisture) to form iron(III) oxide, commonly known as rust.
The Chemistry of Rusting
The chemical reaction involved in rusting is somewhat complex, but a simplified representation is:
4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
This equation shows iron (Fe) reacting with oxygen (O₂) and water (H₂O) to produce iron(III) hydroxide (Fe(OH)₃), which further dehydrates to form various iron oxides, the components of rust.
Observable Changes: Identifying a Chemical Reaction
Several key changes demonstrate that rusting is a chemical change:
- Color Change: The shiny, silvery-grey iron transforms into a reddish-brown, flaky substance—rust. This is a clear visual indication of a new substance being formed.
- Texture Change: The smooth surface of the iron becomes rough and pitted as rust forms, altering its physical texture.
- Loss of Strength and Integrity: Rust weakens the iron, making it brittle and prone to crumbling. The structural integrity of the iron is compromised.
- Irreversibility: Rust cannot easily be converted back into pure iron. The process is essentially irreversible under normal conditions.
- Formation of a New Compound: Iron(III) oxide (rust) is a completely different chemical compound with different properties compared to iron.
Deeper Understanding: Environmental Factors
The rate of rusting is influenced by several environmental factors. The presence of salt water accelerates the process significantly, explaining why rusting is often more severe in coastal areas. High humidity and acidic environments also contribute to faster rust formation. Understanding these factors is crucial in preventing or mitigating rust damage.
3. Baking a Cake: Chemical Reactions in Cooking
Baking a cake offers a fascinating example of a chemical change involving several simultaneous reactions. While the process seems simple, a complex interplay of chemical reactions leads to the final product.
The Chemistry of Baking
Baking a cake involves several key chemical changes:
- Leavening Agents: Baking powder or baking soda, acting as leavening agents, produce carbon dioxide gas when exposed to moisture and heat. This gas expansion creates the air pockets that make the cake light and fluffy. The reaction for baking soda is:
NaHCO₃ (Baking soda) + H⁺ (from an acid in the recipe) → CO₂ (Carbon dioxide) + H₂O (Water) + Na⁺ (Sodium ion)
- Maillard Reaction: The browning of the cake's surface is due to the Maillard reaction, a chemical reaction between amino acids and reducing sugars when exposed to heat. This reaction produces hundreds of different flavor and aroma compounds, contributing to the cake's characteristic color and taste.
- Protein Denaturation: The proteins in eggs and flour undergo denaturation when heated. Their three-dimensional structures unfold, forming a stable network that gives the cake structure and texture.
- Gelatinization of Starch: The starch in flour absorbs water and swells when heated, forming a gel-like structure. This contributes to the cake's texture and moisture content.
Observable Changes: Evidence in the Kitchen
The transformation of the cake batter into a cake is a clear example of a chemical change:
- Change in Texture: The liquid batter transforms into a solid, spongy cake. The changes in consistency are substantial and indicate a transformation of the components.
- Change in Appearance: The color of the cake changes from pale to golden brown due to the Maillard reaction. This visual change shows the formation of new compounds.
- Change in Flavor and Aroma: The mixture of ingredients undergoes a dramatic transformation in taste and smell, producing the characteristic aroma and taste of a baked cake. The formation of new flavor compounds is a significant result of the chemical changes involved.
- Irreversibility: Once baked, you cannot revert the cake back to its original batter form. The chemical reactions are essentially irreversible.
- Gas Formation: The visible air pockets in the cake are a direct result of carbon dioxide production from the leavening agents, demonstrating the role of gas in creating the cake's texture.
Deeper Understanding: Recipe Adjustments
The success of a cake relies heavily on the precise balance of ingredients and the control of temperature and baking time. Adjustments to the recipe can significantly alter the final product, highlighting the sensitivity of the chemical reactions involved.
These three examples – burning a candle, rusting of iron, and baking a cake – demonstrate the diverse nature of chemical changes. Each involves the formation of new substances with different properties, indicating a transformation at a molecular level. Understanding these processes provides a strong foundation for grasping the concepts of chemical reactions and their significance in our everyday lives. By observing the observable changes and understanding the underlying chemistry, we can gain a deeper appreciation for the intricate world of chemical transformations.
Latest Posts
Latest Posts
-
Subject And Predicate Exercises With Answers
Apr 02, 2025
-
Greater Than Less Than Decimal Calculator
Apr 02, 2025
-
How Many Times Does 4 Go Into 72
Apr 02, 2025
-
How Do You Factorize An Equation
Apr 02, 2025
-
Can Triangle Have 2 Right Angles
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are Three Examples Of A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
