What Are The Two Parts Of An Atom
Juapaving
Apr 01, 2025 · 6 min read
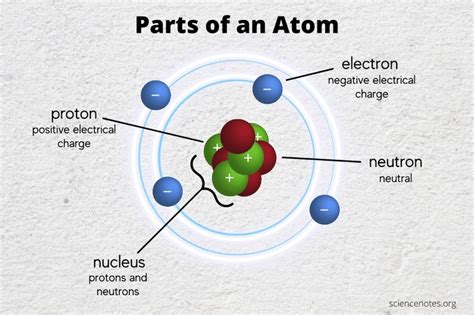
Table of Contents
Delving Deep: The Two Main Parts of an Atom
Atoms, the fundamental building blocks of all matter, are incredibly tiny, yet incredibly complex. Understanding their structure is key to understanding the world around us, from the smallest living organisms to the largest stars. While the atom's structure has been refined and expanded upon over time with the discovery of subatomic particles, at its most basic level, an atom can be understood as having two primary parts: the nucleus and the electron cloud. Let's delve into each component in detail.
1. The Nucleus: The Atom's Dense Core
The nucleus, often described as the "brain" of the atom, is located at its center. It's incredibly small, occupying only a tiny fraction of the atom's overall volume, yet it houses the majority of the atom's mass. This dense core consists of two types of subatomic particles: protons and neutrons.
1.1 Protons: Positively Charged Particles
Protons carry a single positive electrical charge (+1). The number of protons in an atom's nucleus defines its atomic number and determines what element it is. For example, an atom with one proton is hydrogen, an atom with two protons is helium, and so on. This number is crucial in differentiating elements and understanding their chemical properties. The positive charge of protons plays a vital role in the atom's interactions with other atoms and molecules. The strong nuclear force, one of the fundamental forces of nature, binds protons together within the nucleus, overcoming the electrostatic repulsion between their like charges.
1.2 Neutrons: Neutral Particles
Neutrons, as their name suggests, carry no electrical charge (0). They are slightly more massive than protons. The number of neutrons in an atom's nucleus can vary, even for atoms of the same element. These variations create different isotopes of the same element. Isotopes have the same number of protons but a different number of neutrons. Some isotopes are stable, while others are radioactive, meaning their nuclei spontaneously decay over time, emitting radiation. This radioactive decay is used in various applications, from medical imaging to dating ancient artifacts. Neutrons contribute to the overall mass of the nucleus and play a crucial role in stabilizing it against the electrostatic repulsion between protons. The strong nuclear force also binds neutrons to protons and other neutrons.
1.3 Isotopes and their Significance
The concept of isotopes is crucial for understanding the diversity within elements. Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties. Some isotopes are stable, existing indefinitely, while others are unstable or radioactive. These radioactive isotopes decay over time, emitting particles and energy. This decay is characterized by a specific half-life, which is the time it takes for half of the atoms in a sample to decay.
Radioactive isotopes find numerous applications across various fields:
- Medical Imaging: Radioactive tracers, incorporated into specific molecules, are used to visualize internal organs and processes in the body.
- Cancer Treatment: Radiation therapy uses radioactive isotopes to target and destroy cancerous cells.
- Archaeological Dating: Radiocarbon dating utilizes the decay of carbon-14 to estimate the age of ancient organic materials.
- Industrial Applications: Radioactive isotopes are employed in various industrial processes, including gauging thickness, detecting leaks, and sterilization.
2. The Electron Cloud: A Realm of Probability
Unlike the nucleus, the electron cloud is not a solid, defined structure. Instead, it's a region surrounding the nucleus where electrons are most likely to be found. Electrons are negatively charged particles (-1) with significantly less mass than protons and neutrons. Their movement is governed by the principles of quantum mechanics, which describe their behavior in terms of probability rather than precise locations.
2.1 Electron Orbitals and Energy Levels
Electrons don't orbit the nucleus in neat, circular paths like planets around a sun. Instead, they occupy regions of space called orbitals, which are defined by their energy levels and shapes. These orbitals are described by quantum numbers, which specify the electron's energy, angular momentum, and magnetic moment. Electrons occupy orbitals in a specific order, filling lower energy levels before moving to higher ones. The arrangement of electrons in an atom's orbitals determines its chemical behavior.
2.2 Electron Shells and Subshells
Electrons are organized into shells, which represent different energy levels. Shells are further divided into subshells, each containing a specific number of orbitals. The number of electrons that a shell can hold increases as you move further away from the nucleus. The outermost shell, also known as the valence shell, contains the valence electrons, which are responsible for the atom's chemical bonding properties. The number of valence electrons determines an element's reactivity and the types of bonds it can form.
2.3 Electron Configuration and Chemical Properties
The electron configuration of an atom describes the arrangement of electrons in its various shells and subshells. This configuration dictates the atom's chemical behavior. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing electrons with other atoms. This process leads to the formation of chemical bonds, which hold atoms together to form molecules and compounds. For instance, atoms with a nearly full valence shell tend to gain electrons, while atoms with only a few valence electrons tend to lose them. This tendency drives chemical reactions and determines the properties of substances.
2.4 Quantum Mechanics and Electron Behavior
The behavior of electrons is governed by the principles of quantum mechanics. This means that we can't precisely determine both the position and momentum of an electron simultaneously. This uncertainty principle is a fundamental aspect of quantum mechanics and implies that the electron's location is described by a probability distribution rather than a precise trajectory. This probability distribution is visualized as the electron cloud, representing the regions of space where electrons are most likely to be found.
The Interplay Between Nucleus and Electron Cloud: Atomic Stability and Reactivity
The nucleus and electron cloud are inextricably linked. The positive charge of the protons in the nucleus attracts the negatively charged electrons in the cloud, holding the atom together. The balance between the positive charge of the nucleus and the negative charge of the electrons determines the atom's overall electrical neutrality. The arrangement of electrons in the electron cloud, particularly the valence electrons, governs the atom's chemical reactivity. Atoms with incomplete valence shells tend to react with other atoms to achieve a stable configuration, usually a full valence shell. This tendency drives the formation of chemical bonds, leading to the vast array of molecules and compounds that make up our world.
Conclusion: A Microscopic Universe
The seemingly simple concept of an atom, with its two primary parts – the nucleus and the electron cloud – opens up a world of incredible complexity. From the strong nuclear force holding the nucleus together to the probabilistic nature of the electron cloud, exploring the atom reveals the fundamental principles governing the universe. Understanding the interplay between protons, neutrons, and electrons is key to comprehending the properties of matter, driving scientific advancements across diverse fields, from medicine and technology to materials science and astrophysics. The journey into the atom continues to reveal more wonders, pushing the boundaries of our understanding of the universe's building blocks.
Latest Posts
Latest Posts
-
Least Common Multiple Of 20 And 30
Apr 02, 2025
-
Difference Between Starch Cellulose And Glycogen
Apr 02, 2025
-
What Is The Primary Function Of The Excretory System
Apr 02, 2025
-
How To Write 1300 On A Check
Apr 02, 2025
-
Examples Of Farewell Speech For Retirement
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are The Two Parts Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
