What Are The P Block Elements
Juapaving
Mar 21, 2025 · 6 min read
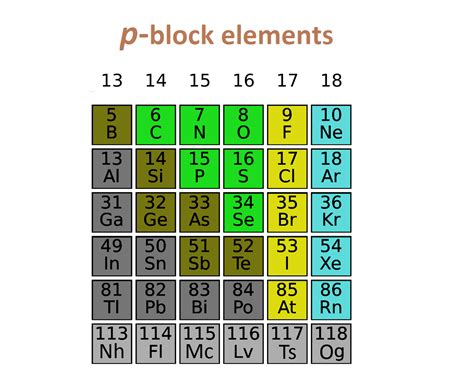
Table of Contents
What are the p-block Elements? A Deep Dive into the Periodic Table's Colorful Corner
The periodic table, that iconic chart of chemical elements, is organized into blocks based on the electronic configuration of their atoms. One particularly vibrant and diverse section is the p-block, home to a fascinating array of elements crucial to life, technology, and everything in between. This article will delve deep into the p-block elements, exploring their properties, trends, and applications, shedding light on their significant role in our world.
Understanding Electronic Configuration: The Key to p-Block Identity
Before we dive into the specifics of the p-block elements, let's establish a foundational understanding of their defining characteristic: their electronic configuration. The p-block comprises elements where the last electron enters a p subshell. Each p subshell can hold up to six electrons, leading to a wide range of properties and behaviors within the block. This contrasts with the s-block (alkali and alkaline earth metals) and d-block (transition metals), where the last electron fills the s and d subshells, respectively.
The Location and Extent of the p-Block
Located on the right-hand side of the periodic table, the p-block spans Groups 13 to 18. This includes a diverse collection of elements, exhibiting various states of matter at room temperature – from gases (like oxygen and chlorine) to solids (like phosphorus and iodine) and even liquids (like bromine). This variety is a key feature distinguishing the p-block.
Exploring the Groups: Diverse Properties and Applications
The p-block's organization into groups reflects recurring patterns in their properties. Let's explore each group individually:
Group 13: The Boron Family – A Trio of Metalloids and Metals
Group 13, also known as the boron family, is unique for containing both metalloids (elements with properties intermediate between metals and nonmetals) and metals. Boron itself is a metalloid, vital in materials science, particularly in the creation of high-strength, lightweight materials. Aluminum, a highly abundant metal, finds ubiquitous application in packaging, construction, and transportation due to its lightness and corrosion resistance. Gallium, indium, and thallium, the heavier members, exhibit increasingly metallic characteristics, with applications ranging from semiconductors to specialized alloys. The increasing metallic character down the group is a common trend observed in the p-block.
Key Characteristics of Group 13:
- Variable oxidation states: They can exhibit +1 and +3 oxidation states.
- Amphoteric nature: Many compounds show both acidic and basic properties.
- Catalytic activity: Certain compounds act as catalysts in various chemical reactions.
Group 14: The Carbon Family – The Backbone of Life and Technology
Group 14, or the carbon family, is arguably the most significant group in the p-block due to carbon's central role in organic chemistry and life itself. Carbon's unique ability to form strong covalent bonds with itself and other elements leads to an incredible diversity of organic compounds. Silicon, the second member, is a cornerstone of the semiconductor industry, crucial for the creation of microchips and other electronic devices. Germanium, tin, and lead complete the group, exhibiting increasing metallic properties down the group. Tin finds application in coatings and alloys, while lead, despite its toxicity, continues to find niche uses in specialized applications.
Key Characteristics of Group 14:
- Tetravalency: Most members exhibit a +4 oxidation state.
- Catenation: Ability to form long chains and rings of atoms, particularly prominent in carbon.
- Variety of bonding: They exhibit diverse bonding types, including covalent, ionic, and metallic.
Group 15: The Nitrogen Family – Essential for Life and Industrial Processes
Group 15, the nitrogen family, contains elements crucial for life and industrial processes. Nitrogen itself forms a significant component of the atmosphere and is essential for the synthesis of amino acids and proteins. Phosphorus, in various allotropic forms, plays a vital role in biological systems as well as in fertilizers and detergents. Arsenic, antimony, and bismuth complete the group, with applications ranging from semiconductors to pharmaceuticals and alloys. However, several members of this group also exhibit toxicity, highlighting the need for careful handling and application.
Key Characteristics of Group 15:
- Variable oxidation states: They can exhibit a wide range of oxidation states, from -3 to +5.
- Allotropism: Many members exist in different structural forms (allotropes) with varying properties.
- Formation of hydrides: They form hydrides with varying stability.
Group 16: The Oxygen Family – Life's Breath and Industrial Workhorses
Group 16, or the oxygen family (chalcogens), features elements essential for life and industrial processes. Oxygen, arguably the most crucial element for life, plays a central role in respiration and combustion. Sulfur, a vital nutrient, is also crucial in industrial applications, such as the production of sulfuric acid. Selenium and tellurium are less abundant but find uses in semiconductors and specialized alloys. Polonium, the last member, is a radioactive element with limited applications.
Key Characteristics of Group 16:
- Variable oxidation states: They commonly exhibit -2, +2, +4, and +6 oxidation states.
- Formation of oxides: They readily form oxides with varying properties.
- Non-metallic character (mostly): Oxygen and sulfur are nonmetals, while selenium and tellurium are metalloids.
Group 17: The Halogens – Reactive and Versatile
Group 17, the halogens, consists of highly reactive nonmetals. Fluorine, chlorine, bromine, iodine, and astatine display increasingly weaker oxidizing power down the group. Halogens are crucial in numerous industrial applications, ranging from disinfectants (chlorine) to refrigerants and pharmaceuticals (various halogens). Their reactivity stems from their high electronegativity, the ability to attract electrons towards themselves in a chemical bond.
Key Characteristics of Group 17:
- High electronegativity: They have a strong tendency to gain electrons.
- Formation of halides: They react readily with metals to form ionic halides.
- Oxidizing agents: They are powerful oxidizing agents, capable of accepting electrons from other substances.
Group 18: The Noble Gases – Inert and Essential
Group 18, the noble gases (inert gases), comprises elements renowned for their inertness due to their complete outermost electron shells. Helium, neon, argon, krypton, xenon, and radon are all gases under standard conditions. While long considered unreactive, xenon and krypton have shown the ability to form compounds under specific conditions. Noble gases find applications in lighting (neon signs), lasers, and cryogenics (helium).
Key Characteristics of Group 18:
- High ionization energy: They require significant energy to remove an electron.
- Low reactivity: Their stable electronic configuration leads to low reactivity.
- Applications in various fields: They are utilized in diverse areas, from lighting to cryogenics.
Trends in the p-Block: A Systematic Exploration
Several trends emerge as we move down and across the p-block:
- Atomic radius: Atomic size increases down a group and decreases across a period.
- Ionization energy: Ionization energy decreases down a group and increases across a period.
- Electronegativity: Electronegativity decreases down a group and increases across a period.
- Metallic character: Metallic character generally increases down a group and decreases across a period.
Conclusion: The p-Block's Enduring Significance
The p-block elements constitute a diverse and crucial segment of the periodic table. From the life-sustaining roles of carbon, nitrogen, and oxygen to the technological importance of silicon and the halogens, these elements shape our world in countless ways. Understanding their properties, trends, and applications is fundamental to advancements in diverse fields, from materials science and medicine to electronics and energy production. Further research continues to uncover the remarkable properties and potential applications of these fascinating elements. The p-block, in essence, represents a testament to the richness and complexity of the chemical world and its impact on our daily lives.
Latest Posts
Latest Posts
-
What Are The Pros Of Fossil Fuels
Mar 27, 2025
-
An Object Following A Straight Line Path At Constant Speed
Mar 27, 2025
-
Is Ice Cream Melting A Physical Change
Mar 27, 2025
-
Why Does Electronegativity Increase From Left To Right
Mar 27, 2025
-
What Is The Lcm Of 25 And 35
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Are The P Block Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
