What Are The Horizontal Rows On The Periodic Table Called
Juapaving
Mar 15, 2025 · 6 min read
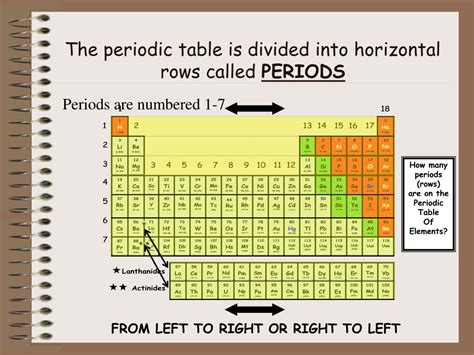
Table of Contents
What Are the Horizontal Rows on the Periodic Table Called? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While most are familiar with the vertical columns, called groups or families, many are less certain about the horizontal rows. This comprehensive guide delves into the answer to the question: what are the horizontal rows on the periodic table called? We'll explore not only the name – periods – but also the crucial role they play in understanding the structure of atoms and the behavior of elements.
Understanding Periods: The Horizontal Organization of Elements
The horizontal rows in the periodic table are known as periods. Each period represents a principal energy level or shell in an atom. As you move across a period from left to right, the number of electrons in the outermost shell increases. This systematic addition of electrons leads to predictable changes in the chemical and physical properties of the elements within that period.
Period 1: The Simplest Beginnings
The first period is remarkably short, containing only two elements: hydrogen (H) and helium (He). These elements have only one electron shell, and their electrons occupy the 1s orbital. Hydrogen, with one electron, is highly reactive, while helium, with a full electron shell, is an extremely stable and inert noble gas.
Period 2 and Period 3: Expanding the Electron Shells
Period 2 and Period 3, each containing eight elements, introduce the concept of subshells. While Period 1 only utilizes the 1s orbital, Period 2 includes both the 2s and 2p orbitals. This expansion leads to a greater diversity in the properties of the elements. We see the transition from highly reactive alkali metals (like lithium (Li) and sodium (Na)) to increasingly electronegative nonmetals (such as oxygen (O) and chlorine (Cl)), culminating in the noble gas neon (Ne) and argon (Ar). Period 3 follows a similar pattern, with elements exhibiting analogous properties but with slightly altered characteristics due to the increased number of electron shells.
Periods 4-7: The Complexity of Electron Configuration
Periods 4-7 become increasingly complex, showcasing the filling of additional electron subshells (3d, 4d, 5d, 4f, 5f). This leads to the introduction of transition metals, characterized by their ability to exhibit multiple oxidation states and form colorful compounds. The lanthanides and actinides, found in periods 6 and 7 respectively, further demonstrate the intricate electron configurations within these longer periods.
-
Transition metals: These elements are located in the middle of the periodic table, displaying unique properties like variable oxidation states and catalytic activity. Examples include iron (Fe), copper (Cu), and platinum (Pt).
-
Lanthanides (Rare Earth Elements): These elements are characterized by the filling of the 4f subshell. They exhibit similar chemical properties, making separation challenging.
-
Actinides: These radioactive elements fill the 5f subshell, showcasing complex nuclear behavior and varied applications in nuclear technology and research.
The Significance of Periodicity in Chemical Properties
The arrangement of elements into periods reflects the periodic recurrence of chemical properties. This periodicity is directly related to the electronic configuration of the atoms. As we move across a period, the number of valence electrons – electrons in the outermost shell – increases. These valence electrons are primarily responsible for chemical bonding and reactivity.
Trends Across a Period: A Glimpse into Reactivity
Several important trends emerge as we traverse a period:
-
Atomic Radius: Generally decreases across a period. The increasing nuclear charge pulls the electrons closer to the nucleus.
-
Ionization Energy: Generally increases across a period. It becomes increasingly difficult to remove an electron from an atom as the nuclear charge increases.
-
Electronegativity: Generally increases across a period. Atoms with a higher electronegativity have a greater tendency to attract electrons in a chemical bond.
-
Metallic Character: Generally decreases across a period. Elements on the left side of a period tend to be more metallic, while those on the right are more non-metallic.
These trends explain why elements within the same period often exhibit strikingly different chemical behavior despite their proximity in the periodic table. The progressive filling of electron shells and the concomitant changes in atomic properties are clearly reflected in the arrangement of elements in periods.
Periods and Valence Electrons: The Key to Chemical Bonding
The number of valence electrons in an atom largely determines its chemical reactivity and the types of bonds it can form. Elements within the same period, while differing in their overall electron configuration, may share similar valence electron counts, leading to some similarities in their chemical behavior. However, the increasing nuclear charge significantly impacts their properties, influencing electronegativity, ionization energy, and the likelihood of forming different types of chemical bonds (ionic, covalent, or metallic).
Predicting Chemical Behavior Based on Period and Group
The combination of period and group provides a powerful tool for predicting the chemical behavior of an element. Knowing the period reveals the number of electron shells, influencing the size and reactivity of the atom. The group indicates the number of valence electrons, dictating the preferred bonding patterns and chemical interactions. This combined information allows for a deeper understanding of an element's properties and how it interacts with other elements.
Beyond the Basics: The Exceptions and Nuances of Periodic Trends
While the general trends described above provide a valuable framework for understanding periodic properties, there are exceptions and nuances that require consideration. These deviations often stem from complex electron interactions within the atom, including electron shielding, electron-electron repulsion, and the stability of half-filled or fully-filled subshells.
For instance, the ionization energy doesn't always strictly increase across a period. Sometimes, slight irregularities can be observed due to the stability associated with half-filled or fully-filled subshells. Similarly, the atomic radius doesn't always decrease smoothly. The influence of electron shielding can lead to minor deviations from the expected trend.
Understanding these exceptions requires a more in-depth exploration of atomic structure and quantum mechanics. However, the basic principles governing periodic trends remain a cornerstone of understanding the chemical properties of the elements.
The Practical Applications of Understanding Periods
The concept of periods in the periodic table is not merely an academic exercise. It has far-reaching implications in various scientific and technological fields.
-
Material Science: Understanding the periodic trends within periods is crucial for designing new materials with specific properties. By carefully selecting elements from different periods and groups, scientists can tailor materials for diverse applications, such as high-strength alloys, semiconductors, and superconductors.
-
Chemical Synthesis: The knowledge of how elements in the same period behave differently in chemical reactions allows for predicting the outcome of synthesis processes and developing optimized reaction conditions.
-
Catalysis: Many transition metals, found across multiple periods, are exceptional catalysts. Understanding their electronic structure and their placement within the periodic table helps in developing new catalysts for industrial processes and green chemistry applications.
Conclusion: Periods – The Foundation of Chemical Understanding
In conclusion, the horizontal rows of the periodic table are called periods. Each period represents a principal energy level, signifying the systematic addition of electrons into an atom's electron shells. The arrangement of elements into periods reflects the periodic recurrence of their chemical and physical properties, providing a powerful tool for understanding the behavior of elements and predicting their interactions. The significance of periods extends beyond theoretical chemistry, with practical applications spanning material science, chemical synthesis, and catalysis. Therefore, comprehending the concept of periods is fundamental for anyone seeking a deep understanding of chemistry and its vast applications. From the simple elegance of Period 1 to the complexity of the lanthanides and actinides, periods offer an essential framework for organizing and interpreting the behavior of the elements.
Latest Posts
Latest Posts
-
What Is The Prime Factorization Of 200
Mar 15, 2025
-
The Si Unit Of Energy Is The
Mar 15, 2025
-
Is Water A Compound Mixture Or Element
Mar 15, 2025
-
What Is The Least Common Multiple Of 12 And 8
Mar 15, 2025
-
What Are The Factors Of 86
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Are The Horizontal Rows On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
