The Si Unit Of Energy Is The
Juapaving
Mar 15, 2025 · 6 min read
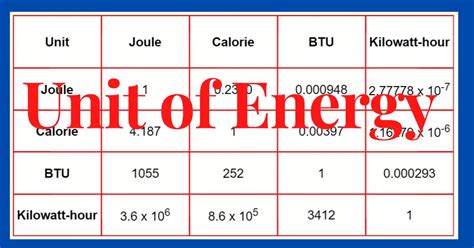
Table of Contents
The SI Unit of Energy Is the Joule: A Deep Dive into Energy Measurement
The fundamental concept of energy underpins our understanding of the physical world. From the smallest subatomic particles to the largest celestial bodies, energy drives change and interaction. Precisely measuring and quantifying this vital force requires a standardized unit, and that unit, within the International System of Units (SI), is the joule (J). This article will delve deep into the joule, exploring its definition, applications, conversions, and significance in various scientific fields.
Understanding the Joule: Definition and Derivation
The joule, named after the 19th-century English physicist James Prescott Joule, is defined as the energy transferred to (or work done on) an object when a force of one newton acts on that object in the direction of its motion through a distance of one meter. This seemingly simple definition encapsulates a profound relationship between force, distance, and energy.
Mathematically, this can be expressed as:
Energy (Joules) = Force (Newtons) × Distance (Meters)
This equation highlights the fundamental connection between mechanical work and energy. When we exert a force to move an object, we are transferring energy to that object. The amount of energy transferred is directly proportional to both the force applied and the distance over which the force is applied.
The Joule in Different Contexts: Beyond Mechanical Work
While the initial definition focuses on mechanical work, the joule's applicability extends far beyond this realm. It's a versatile unit used to quantify energy in diverse forms, including:
-
Thermal Energy (Heat): The joule is used to measure the amount of heat transferred to or from a system. This is crucial in thermodynamics, where understanding heat transfer is paramount. For example, the specific heat capacity of a substance is often expressed in joules per kilogram-kelvin (J/kg·K), indicating the amount of energy required to raise the temperature of one kilogram of the substance by one Kelvin.
-
Electrical Energy: The energy consumed by an electrical appliance is also measured in joules. Power, measured in watts (W), represents the rate of energy transfer (joules per second), so the total energy consumed is the power multiplied by the time the appliance is used. For instance, a 100-watt light bulb operating for one hour consumes 360,000 joules (100 W × 3600 seconds).
-
Chemical Energy: The energy stored in chemical bonds can be quantified in joules. This is especially relevant in fields like chemistry and biochemistry, where understanding the energy changes during chemical reactions is vital. The energy released or absorbed during a reaction is often expressed in kilojoules (kJ) or megajoules (MJ).
-
Nuclear Energy: The immense energy released during nuclear reactions, such as fission and fusion, is also measured in joules. The energy released in these processes is significantly larger than in chemical reactions, often expressed in gigajoules (GJ) or even terajoules (TJ).
-
Radiant Energy (Light): The energy carried by electromagnetic radiation, including light, is also measured in joules. The energy of a photon is directly proportional to its frequency, and this relationship is fundamental to quantum mechanics. The intensity of light, or radiant flux, can be expressed in watts per square meter (W/m²), representing the energy flow per unit area per unit time.
Practical Applications of the Joule
The versatility of the joule makes it indispensable across numerous scientific and engineering disciplines:
-
Physics: From classical mechanics to quantum mechanics, the joule is essential for quantifying energy in various physical phenomena. It's used in calculations involving kinetic energy, potential energy, work, power, and heat transfer.
-
Engineering: In mechanical, electrical, and chemical engineering, the joule is crucial for designing efficient systems and processes. It's used to calculate energy consumption, energy efficiency, and energy losses in various applications.
-
Chemistry: The joule plays a crucial role in thermochemistry, where it's used to measure the heat absorbed or released during chemical reactions. This helps determine the feasibility and spontaneity of reactions.
-
Environmental Science: The joule is used to quantify energy consumption and energy efficiency in environmental studies. It helps assess the environmental impact of different energy sources and technologies.
-
Medicine: In medical imaging techniques like MRI (Magnetic Resonance Imaging), the energy used to generate the magnetic fields is measured in joules.
Conversions and Prefixes: Working with Different Scales of Energy
The joule, like other SI units, employs prefixes to represent different orders of magnitude. This allows us to work conveniently with extremely large or small energy values. Common prefixes include:
- kilojoule (kJ): 1 kJ = 1000 J
- megajoule (MJ): 1 MJ = 1,000,000 J
- gigajoule (GJ): 1 GJ = 1,000,000,000 J
- terajoule (TJ): 1 TJ = 1,000,000,000,000 J
- millijoule (mJ): 1 mJ = 0.001 J
- microjoule (µJ): 1 µJ = 0.000001 J
These prefixes make it easier to handle energy values ranging from the minuscule energy changes in chemical reactions to the enormous energy released in nuclear explosions. Conversions between different units are straightforward, using simple multiplicative factors.
The Joule and Other Energy Units: Understanding the Relationships
While the joule is the standard SI unit, other energy units exist, often used in specific contexts. It's important to understand the relationships between these units for accurate conversions:
-
Calorie (cal): The calorie is a unit of energy commonly used in nutrition and dietetics. One calorie is approximately equal to 4.184 joules.
-
Kilocalorie (kcal) or Calorie (Cal): Often used interchangeably with "Calorie" (with a capital "C"), the kilocalorie is 1000 calories and is equal to approximately 4184 joules. This is the unit often found on food labels.
-
British Thermal Unit (BTU): The BTU is a unit of energy commonly used in the United States to measure heating and cooling capacity. One BTU is approximately equal to 1055 joules.
-
Electronvolt (eV): The electronvolt is a unit of energy commonly used in atomic and nuclear physics. It represents the energy gained by an electron when it is accelerated through a potential difference of one volt. One electronvolt is equal to approximately 1.602 × 10⁻¹⁹ joules.
Knowing these conversions is vital for interpreting energy values in different contexts and accurately comparing them.
The Joule's Significance in Scientific Advancements
The consistent use of the joule has been instrumental in advancing scientific understanding and technological innovation:
-
Improved Energy Efficiency: The precise quantification of energy using the joule allows engineers to design more efficient machines, appliances, and processes, reducing energy waste and promoting sustainability.
-
Advancements in Energy Technologies: Understanding energy conversions and transformations at the joule level has led to breakthroughs in renewable energy technologies, including solar power, wind power, and geothermal energy.
-
Progress in Fundamental Physics: The joule's role in quantifying energy in various physical processes has been critical in advancing our understanding of fundamental physical laws and theories.
Conclusion: The Joule – A Universal Language of Energy
The joule, as the SI unit of energy, serves as a universal language for quantifying and comparing energy in diverse contexts. Its consistent application across scientific disciplines fosters a deeper understanding of energy transformations, promotes efficient energy use, and facilitates advancements in technology and scientific knowledge. The widespread adoption of the joule has undeniably shaped our modern world, paving the way for more efficient, sustainable, and technologically advanced systems. From the smallest atomic interactions to the grand scale of astrophysical phenomena, the joule provides the essential framework for accurately measuring and interpreting the boundless energy that drives our universe.
Latest Posts
Latest Posts
-
What Is The Opposite Word Of Cruel
May 09, 2025
-
When There Is A Decrease In Both Demand And Supply
May 09, 2025
-
How To Separate Sand From Iron Filings
May 09, 2025
-
Group Of Cells With Similar Structure And Function
May 09, 2025
-
What Is The Stem Of A Pumpkin Called
May 09, 2025
Related Post
Thank you for visiting our website which covers about The Si Unit Of Energy Is The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.