What Are The Characteristics Of Covalent Compounds
Juapaving
Mar 26, 2025 · 7 min read
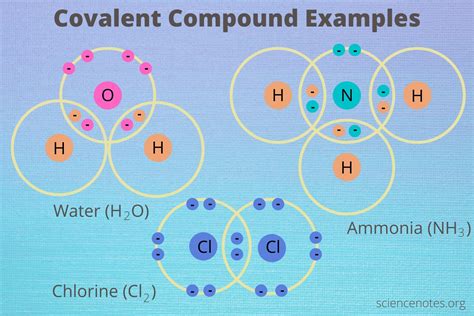
Table of Contents
What are the Characteristics of Covalent Compounds? A Deep Dive
Covalent compounds, also known as molecular compounds, are formed when atoms share electrons to achieve a stable electron configuration, typically resembling a noble gas. Understanding their characteristics is crucial in various fields, from chemistry and materials science to biology and medicine. This comprehensive guide delves into the key properties of covalent compounds, exploring their diverse nature and the reasons behind their unique behaviors.
Physical Properties of Covalent Compounds
The physical properties of covalent compounds are significantly influenced by the strength of the covalent bonds holding the atoms together, as well as the intermolecular forces present between molecules. These properties contrast sharply with those of ionic compounds.
1. Low Melting and Boiling Points:
Generally, covalent compounds have relatively low melting and boiling points. This is because the forces of attraction between individual molecules (intermolecular forces) are significantly weaker than the strong electrostatic forces holding ions together in ionic compounds. Only a small amount of energy is needed to overcome these weaker intermolecular forces, leading to lower melting and boiling points. For example, water (H₂O) boils at 100°C, while sodium chloride (NaCl), an ionic compound, boils at 1413°C.
2. Poor Electrical Conductivity:
Covalent compounds are typically poor conductors of electricity in both solid and liquid states. This is because, unlike ionic compounds, they do not have freely moving charged particles (ions) to carry the electric current. In the solid state, the molecules are held together by weak intermolecular forces, preventing the movement of electrons. Even when melted, the covalent bonds remain intact, and there are no free ions or electrons to conduct electricity. Exceptions exist, such as graphite, where delocalized electrons allow conductivity.
3. Low Solubility in Water:
While some covalent compounds are soluble in water, many are not. This solubility depends on the polarity of the molecule. Polar covalent molecules, which have a slightly positive and slightly negative end due to unequal sharing of electrons, can interact with polar water molecules through dipole-dipole interactions and hydrogen bonding, leading to solubility. Nonpolar covalent molecules, however, do not interact effectively with water molecules, resulting in low solubility. For instance, sugar (a polar covalent compound) dissolves readily in water, while oil (a nonpolar covalent compound) does not.
4. Volatility:
Many covalent compounds are volatile, meaning they readily change from liquid to gas at relatively low temperatures. This is again due to the weak intermolecular forces. Less energy is required to overcome these forces and allow molecules to escape into the gaseous phase. Examples of volatile covalent compounds include ethanol and acetone.
5. Often Insoluble in Polar Solvents:
Conversely to their solubility in water, non-polar covalent compounds are typically insoluble in polar solvents. The principle of "like dissolves like" applies here. Non-polar substances dissolve best in non-polar solvents, while polar substances dissolve best in polar solvents.
6. Brittle Nature (in solid state):
While not a universal characteristic, some solid covalent compounds can be brittle. This is because the covalent bonds within the molecules are strong, but the forces between molecules are weak. When stress is applied, these weak intermolecular forces break easily, leading to fracturing.
Chemical Properties of Covalent Compounds
The chemical properties of covalent compounds are governed by the nature of the covalent bonds and the reactivity of the constituent atoms.
1. Lower Reactivity Compared to Ionic Compounds:
Generally, covalent compounds exhibit lower reactivity than ionic compounds. The shared electrons in covalent bonds are strongly held between the atoms, making them less readily available for reactions. This contrasts with ionic compounds, where ions are relatively easily separated and involved in reactions.
2. Formation of Complex Molecules:
Covalent bonding allows for the formation of large and complex molecules. Carbon, with its ability to form four covalent bonds, plays a crucial role in the formation of long chains and complex structures found in organic compounds, including proteins, carbohydrates, and DNA.
3. Diverse Range of Reactions:
Covalent compounds participate in a wide variety of reactions, including combustion, substitution, addition, elimination, and condensation reactions. The specific reaction a covalent compound undergoes depends on its structure and the presence of functional groups.
4. Reaction Rates:
The rates of reactions involving covalent compounds can vary widely depending on factors such as temperature, concentration, presence of catalysts, and the nature of the reactants.
Types of Covalent Bonds
Understanding the different types of covalent bonds further illuminates the characteristics of covalent compounds.
1. Single Covalent Bonds:
A single covalent bond involves the sharing of one pair of electrons between two atoms. This results in a relatively weaker bond compared to double or triple bonds. For example, the bond in methane (CH₄) is a single covalent bond.
2. Double Covalent Bonds:
A double covalent bond involves the sharing of two pairs of electrons between two atoms. This leads to a stronger bond and shorter bond length compared to a single bond. Ethylene (C₂H₄) contains a double covalent bond between the carbon atoms.
3. Triple Covalent Bonds:
A triple covalent bond involves the sharing of three pairs of electrons between two atoms. This represents the strongest type of covalent bond, with the shortest bond length. Acetylene (C₂H₂) contains a triple covalent bond between the carbon atoms.
4. Polar Covalent Bonds:
In a polar covalent bond, the electrons are not shared equally between the two atoms. This is due to differences in electronegativity, which is the ability of an atom to attract electrons towards itself. The more electronegative atom will pull the electrons closer, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. Water (H₂O) is a classic example of a molecule with polar covalent bonds.
5. Nonpolar Covalent Bonds:
In a nonpolar covalent bond, the electrons are shared equally between two atoms. This occurs when the atoms have similar electronegativity values. Examples include the bonds in diatomic molecules like oxygen (O₂) and nitrogen (N₂).
Intermolecular Forces
The properties of covalent compounds are also significantly affected by the intermolecular forces present between molecules. These forces are much weaker than covalent bonds but still play a critical role.
1. London Dispersion Forces:
These are weak forces present between all molecules, regardless of polarity. They arise from temporary fluctuations in electron distribution, creating temporary dipoles.
2. Dipole-Dipole Forces:
These forces occur between polar molecules due to the attraction between the positive end of one molecule and the negative end of another.
3. Hydrogen Bonding:
This is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. Hydrogen bonding is a relatively strong intermolecular force.
Examples of Covalent Compounds
Covalent compounds are incredibly diverse and are found in numerous applications. Here are a few notable examples:
- Water (H₂O): Essential for life, water exhibits many unique properties due to its polar nature and strong hydrogen bonding.
- Carbon Dioxide (CO₂): A greenhouse gas crucial in the Earth's climate system.
- Glucose (C₆H₁₂O₆): A simple sugar, a vital energy source for living organisms.
- Methane (CH₄): A potent greenhouse gas and a major component of natural gas.
- Ethanol (C₂H₅OH): A common alcohol used as a solvent and fuel.
- Proteins: Large, complex molecules essential for biological functions.
- DNA and RNA: Nucleic acids responsible for genetic information storage and transfer.
- Plastics: Many plastics are composed of long chains of covalently bonded carbon atoms.
Conclusion
The characteristics of covalent compounds are multifaceted and depend on a complex interplay of factors, including the strength of covalent bonds, the type of covalent bonds, the presence of intermolecular forces, and the overall molecular structure. Their low melting and boiling points, poor electrical conductivity, and diverse reactivity contribute to their widespread presence and importance in various aspects of our lives, from the natural world to advanced technologies. Understanding these characteristics is vital for anyone seeking a deeper comprehension of chemistry and its applications.
Latest Posts
Latest Posts
-
What Are The Factors For 86
Mar 26, 2025
-
Electric Field Due To Infinite Line Charge
Mar 26, 2025
-
How Many Chromosomes Does Gametes Have
Mar 26, 2025
-
Whats The Greatest Common Factor Of 12 And 18
Mar 26, 2025
-
Is 34 A Prime Or Composite Number
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Are The Characteristics Of Covalent Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
