What Are Horizontal Rows On The Periodic Table Called
Juapaving
Mar 09, 2025 · 6 min read
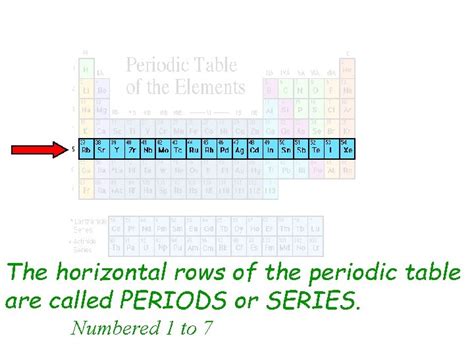
Table of Contents
What Are Horizontal Rows on the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. Understanding its structure is crucial for grasping the behavior of matter. One of the fundamental aspects of this structure lies in its arrangement: the horizontal rows and vertical columns. This article will delve into the question: what are horizontal rows on the periodic table called? and explore their significance in understanding the properties and trends within the elements.
Understanding the Periodic Table's Organization
The periodic table isn't just a random arrangement of elements; it's a carefully constructed system reflecting fundamental relationships between them. Elements are arranged in increasing order of their atomic number, which represents the number of protons in their nucleus. This arrangement reveals recurring patterns in their physical and chemical properties. These patterns are captured by the table's horizontal and vertical organization.
Vertical Columns: Groups or Families
The vertical columns are known as groups or families. Elements within the same group share similar chemical properties because they have the same number of valence electrons—the electrons in their outermost shell. These valence electrons are primarily responsible for chemical bonding and reactivity. For instance, Group 18, the noble gases, are known for their inertness due to their full valence shells.
Horizontal Rows: Periods
Now, let's address the core question of this article: What are horizontal rows on the periodic table called? The answer is periods. Each period represents a principal energy level or shell in an atom. As you move across a period from left to right, the number of electrons in the outermost shell increases. This increase in electrons directly influences the element's properties, leading to observable trends.
The Significance of Periods in Understanding Elemental Properties
Periods are not merely horizontal lines; they represent crucial steps in understanding the behavior of elements. The number of the period corresponds to the highest principal quantum number (n) of the electrons in an atom's ground state. This quantum number defines the electron's energy level and distance from the nucleus.
Trends Across a Period
As we traverse a period, several key trends in properties emerge:
-
Atomic Radius: Generally, atomic radius decreases across a period. This is because the number of protons increases, increasing the positive charge in the nucleus, which attracts the electrons more strongly, pulling them closer.
-
Ionization Energy: Ionization energy, the energy required to remove an electron, increases across a period. The stronger nuclear charge makes it harder to remove an electron.
-
Electron Affinity: Electron affinity, the energy change when an electron is added to an atom, generally increases across a period. The increased nuclear charge makes it more favorable to accept an electron.
-
Electronegativity: Electronegativity, an atom's ability to attract electrons in a chemical bond, increases across a period. This is a direct consequence of the increasing nuclear charge.
-
Metallic Character: Metallic character, the tendency to lose electrons and form positive ions, decreases across a period. Elements on the left side of a period are typically metals, while those on the right are nonmetals.
Variations and Exceptions Within Trends
It's important to note that these trends are general observations. There can be exceptions and subtle variations due to factors such as electron shielding and electron-electron repulsions. However, understanding the general trends provides a valuable framework for predicting the chemical behavior of elements.
Periods and the Electronic Configuration of Elements
The arrangement of elements within periods directly reflects their electronic configuration. The number of elements in each period is determined by the number of electrons that can occupy the sublevels within a given principal energy level.
Period 1: The Simplest Period
Period 1 contains only two elements: hydrogen (H) and helium (He). These elements have electrons only in the first principal energy level (n=1), which can hold a maximum of two electrons.
Period 2 and 3: Expanding Electron Shells
Period 2 and 3 contain eight elements each. This is because the second and third principal energy levels have four sublevels (s and p) which can accommodate a total of eight electrons.
Period 4 and Beyond: The Complexity of d and f Orbitals
Periods 4 and beyond become more complex with the introduction of d and f orbitals. These orbitals can hold significantly more electrons, resulting in longer periods. The presence of d and f orbitals leads to the transition metals (d-block) and inner transition metals (f-block) within the periodic table.
The Significance of Periods in Chemical Reactions
The position of an element within a period influences its reactivity and the types of chemical bonds it forms.
Reactivity Trends
Elements within the same period exhibit varying reactivity depending on their position and electron configuration. For example, alkali metals (Group 1) in a period are highly reactive, readily losing one electron to achieve a stable electron configuration. Halogens (Group 17), on the other hand, are also reactive but tend to gain one electron to achieve a stable octet. Noble gases (Group 18) are exceptionally unreactive due to their full valence electron shells.
Bond Formation
The nature of chemical bonds formed by elements within a period also changes across the period. Elements on the left tend to form ionic bonds by losing electrons, while elements on the right tend to form covalent bonds by sharing electrons. The transition metals exhibit a wide range of oxidation states and can form both ionic and covalent bonds.
Periods and the Prediction of Element Properties
The periodic table, particularly the organization into periods, allows for the prediction of the properties of elements. This predictive power is invaluable in various scientific fields.
Extrapolating Properties
By understanding the trends within a period, scientists can extrapolate the properties of elements that have not yet been synthesized or discovered. This is crucial in materials science, where new materials with desired properties are often sought.
Designing New Materials
Understanding the periodic trends within periods helps in designing new materials with specific properties. For example, the trend in electronegativity can guide the selection of elements to create materials with desired electronic or optical properties.
Conclusion: Periods – The Horizontal Backbone of Chemistry
In summary, the horizontal rows on the periodic table are called periods. They represent the principal energy levels in an atom and are fundamental to understanding the properties and chemical behavior of elements. The trends in atomic radius, ionization energy, electron affinity, electronegativity, and metallic character across a period are valuable tools for predicting and understanding chemical reactivity and the formation of chemical bonds. The organization of elements into periods is a testament to the elegant and powerful system that is the periodic table, providing a framework for understanding the vast complexity of the chemical world. The study of periods provides a crucial foundation for advancements in diverse scientific fields, highlighting their importance in understanding the fundamental building blocks of matter. The predictable nature of the trends across periods is a powerful tool for chemists and materials scientists alike, enabling the design and discovery of novel materials with specific properties. Further exploration of these trends will continue to drive innovation and advancements in chemistry and related fields.
Latest Posts
Latest Posts
-
What Is Oxidized And Reduced In Cellular Respiration
Mar 09, 2025
-
What Is Greater Mb Or Gb
Mar 09, 2025
-
Is Water A Renewable Or A Nonrenewable Resource
Mar 09, 2025
-
Common Factors Of 12 And 9
Mar 09, 2025
-
Are All Rectangles Squares True Or False
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about What Are Horizontal Rows On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
