What Are 3 Components Of A Nucleotide
Juapaving
Mar 07, 2025 · 6 min read
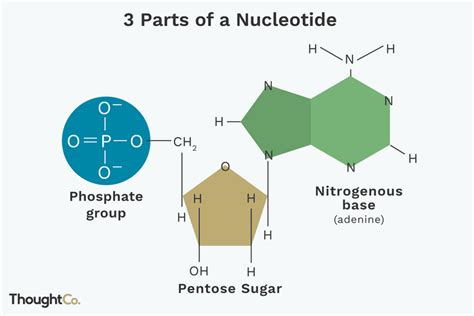
Table of Contents
What are the 3 Components of a Nucleotide? A Deep Dive into the Building Blocks of Life
Nucleotides: the very name conjures images of complex biological machinery, DNA replication, and the intricate dance of life itself. But what exactly are these fundamental molecules? At their core, nucleotides are the building blocks of nucleic acids – DNA and RNA – the molecules responsible for storing and transmitting genetic information. Understanding the three components of a nucleotide is crucial to understanding the foundation of life as we know it. This article will delve deep into these components, exploring their structure, function, and significance in various biological processes.
The Tripartite Nature of Nucleotides: Sugar, Base, and Phosphate
A nucleotide, in its simplest form, is composed of three distinct components:
- A Pentose Sugar: A five-carbon sugar molecule.
- A Nitrogenous Base: A ringed structure containing nitrogen atoms.
- A Phosphate Group: A phosphorus atom bonded to four oxygen atoms.
Let's examine each component in detail:
1. The Pentose Sugar: The Sweet Backbone
The pentose sugar forms the backbone of the nucleotide. There are two primary types of pentose sugars found in nucleotides:
-
Ribose: Found in RNA (ribonucleic acid). Ribose is a five-carbon sugar with a hydroxyl (-OH) group attached to the 2' carbon atom. This hydroxyl group plays a crucial role in RNA's structure and reactivity, making RNA less stable than DNA.
-
Deoxyribose: Found in DNA (deoxyribonucleic acid). Deoxyribose is almost identical to ribose, except it lacks a hydroxyl group at the 2' carbon atom. This seemingly minor difference has profound consequences; the absence of the hydroxyl group makes DNA more stable and better suited for long-term storage of genetic information.
The numbering of the carbon atoms in the pentose sugar is crucial for understanding how the other components attach. The numbering proceeds clockwise around the ring, starting with the carbon atom to the right of the oxygen atom in the ring.
The Importance of the 2' Hydroxyl Group
The presence or absence of the hydroxyl group at the 2' carbon is a key differentiating factor between ribose and deoxyribose, and significantly impacts the properties and functions of RNA and DNA. The 2'-OH group in ribose makes RNA more susceptible to hydrolysis, a chemical reaction that breaks down the molecule. This inherent instability is partly responsible for RNA's shorter lifespan compared to DNA. The 2'-OH group also contributes to RNA's greater flexibility and ability to adopt complex secondary and tertiary structures, crucial for its catalytic roles in certain processes.
2. The Nitrogenous Base: The Information Carrier
The nitrogenous base is the information-carrying component of the nucleotide. It is a nitrogen-containing aromatic heterocyclic ring structure. There are five main nitrogenous bases:
- Adenine (A): A purine base, characterized by a double-ring structure.
- Guanine (G): Another purine base, also with a double-ring structure.
- Cytosine (C): A pyrimidine base, possessing a single-ring structure.
- Thymine (T): A pyrimidine base, found only in DNA.
- Uracil (U): A pyrimidine base, found only in RNA, replacing thymine.
Purines vs. Pyrimidines: Structural Differences and Functional Implications
The division into purines (adenine and guanine) and pyrimidines (cytosine, thymine, and uracil) reflects significant structural differences. Purines possess a larger, double-ring structure, while pyrimidines have a smaller, single-ring structure. This structural difference plays a vital role in the base-pairing interactions that are fundamental to DNA and RNA structure and function. Specifically, purines always pair with pyrimidines (A with T/U and G with C) in a specific arrangement, ensuring the correct distance between the two strands of the double helix.
Base Pairing: The Foundation of Genetic Information
The specific pairing of bases – adenine with thymine (or uracil) and guanine with cytosine – is held together by hydrogen bonds. These hydrogen bonds are relatively weak individually, but their collective strength in a long DNA or RNA molecule provides the necessary stability for the structure. This specific base pairing is the fundamental mechanism behind the replication and transcription of genetic information. The sequence of bases along the nucleic acid chain determines the genetic code.
3. The Phosphate Group: The Energetic Link
The phosphate group is a crucial component of the nucleotide, providing several critical functions:
-
Linkage: The phosphate group links the 5' carbon of one sugar to the 3' carbon of the next sugar, forming the sugar-phosphate backbone of nucleic acids. This linkage is a phosphodiester bond, a strong covalent bond that provides structural stability to the molecule.
-
Energy Currency: Nucleotides containing multiple phosphate groups, such as ATP (adenosine triphosphate), serve as the primary energy currency of cells. The hydrolysis (breaking) of the phosphate bonds releases a significant amount of energy that fuels various cellular processes.
-
Regulation: Phosphorylation (adding a phosphate group) and dephosphorylation (removing a phosphate group) of nucleotides are essential regulatory mechanisms in many cellular processes. These modifications can activate or deactivate enzymes, altering the rate of reactions and controlling cellular function.
ATP: The Cellular Energy Powerhouse
Adenosine triphosphate (ATP) is perhaps the most well-known nucleotide. It is a crucial energy carrier in cells, providing the energy required for a wide range of cellular functions, including muscle contraction, nerve impulse transmission, and protein synthesis. The energy is released through the hydrolysis of the terminal phosphate bond, forming adenosine diphosphate (ADP) and inorganic phosphate (Pi). This process is highly exergonic, meaning it releases a large amount of energy that can be harnessed to drive other endergonic (energy-requiring) reactions.
Nucleotide Structure and Function: A Summary
The three components of a nucleotide – the pentose sugar, the nitrogenous base, and the phosphate group – work together in a remarkably elegant way. The sugar provides the structural backbone, the base carries the genetic information, and the phosphate group links the nucleotides together and provides energy for cellular processes. The specific arrangement of these components determines the type of nucleotide, and the sequence of nucleotides in a nucleic acid chain dictates the genetic code. Understanding the interplay of these three components is essential to understanding the fundamental processes of life.
Beyond the Basics: Variations and Specializations of Nucleotides
While the three core components – sugar, base, and phosphate – define a nucleotide, the vast diversity of biological functions stems from variations within these components.
-
Modifications of the sugar: Beyond ribose and deoxyribose, there are variations like arabinose in certain antibiotics, highlighting the structural flexibility that nature has exploited.
-
Modifications of the bases: Methylation and other chemical modifications of the bases are crucial in regulating gene expression and other cellular processes. These modifications alter the interaction properties of bases, affecting DNA structure and function.
-
Multiple phosphate groups: Nucleotides can have one, two, or three phosphate groups, leading to differences in their energetic capabilities and their role as signaling molecules.
Nucleotides and Diseases: When the Building Blocks Go Wrong
Malfunctions in nucleotide metabolism can have serious consequences, leading to various diseases. Defects in enzymes responsible for nucleotide synthesis or repair can result in genetic instability, contributing to cancer and other genetic disorders. Disruptions in nucleotide salvage pathways, which recycle nucleotides, can also lead to health problems.
Conclusion: The Enduring Importance of Nucleotides
The three components of a nucleotide – the pentose sugar, the nitrogenous base, and the phosphate group – represent a fundamental unit in the architecture of life. Their intricate interactions underpin the storage and transmission of genetic information, the generation of cellular energy, and the regulation of numerous vital processes. Further research continues to unveil the remarkable versatility and complexity of nucleotides, ensuring their central role in our understanding of biology remains firmly established. From the simple elegance of their structure to their profound impact on cellular function, nucleotides remain a cornerstone of life's intricate mechanisms. Their study continues to reveal fascinating insights into the fundamental processes that govern all living organisms.
Latest Posts
Latest Posts
-
Does A Liquid Have A Definite Shape
Mar 09, 2025
-
Bronsted Lowry Acid Vs Lewis Acid
Mar 09, 2025
-
What Is A Factor Of 78
Mar 09, 2025
-
What Is The Prime Factorization Of 91
Mar 09, 2025
-
Is Carbon Metal Nonmetal Or Metalloid
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about What Are 3 Components Of A Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
