Three Physical Properties Of Ionic Compounds
Juapaving
Mar 22, 2025 · 6 min read
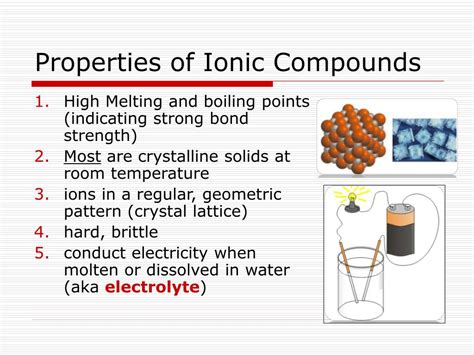
Table of Contents
Three Physical Properties of Ionic Compounds: A Deep Dive
Ionic compounds, formed through the electrostatic attraction between oppositely charged ions, exhibit a fascinating array of physical properties distinctly different from those of covalent compounds. Understanding these properties is crucial in various fields, from materials science and chemistry to geology and medicine. This article delves into three key physical properties of ionic compounds: high melting and boiling points, hardness and brittleness, and electrical conductivity. We'll explore the underlying reasons behind these characteristics and their practical implications.
High Melting and Boiling Points: A Strong Bond Makes a Difference
One of the most prominent physical properties of ionic compounds is their remarkably high melting and boiling points. This characteristic stems directly from the strong electrostatic forces of attraction between the positively charged cations and negatively charged anions within the crystal lattice. These forces, known as ionic bonds, require a significant amount of energy to overcome.
The Strength of Ionic Bonds
The strength of the ionic bond is influenced by several factors:
-
Charge of the ions: Higher charges on the ions lead to stronger electrostatic attraction and thus higher melting and boiling points. For example, magnesium oxide (MgO), with Mg²⁺ and O²⁻ ions, has a much higher melting point than sodium chloride (NaCl), with Na⁺ and Cl⁻ ions.
-
Size of the ions: Smaller ions result in stronger ionic bonds because the distance between the charged nuclei is reduced, increasing the electrostatic attraction. Lithium fluoride (LiF), with smaller ions, has a higher melting point than potassium iodide (KI), with larger ions.
-
Lattice structure: The arrangement of ions in the crystal lattice also affects the overall strength of the ionic bonding. A more tightly packed structure generally leads to a higher melting point.
Overcoming the Bond: Melting and Boiling
To melt an ionic compound, enough energy must be supplied to overcome the strong electrostatic attractions between the ions, allowing them to move more freely. This explains why ionic compounds generally have high melting points, often exceeding several hundred degrees Celsius. Similarly, boiling an ionic compound requires even more energy to completely separate the ions into the gaseous phase. Therefore, ionic compounds also tend to have high boiling points.
Practical Implications
The high melting and boiling points of ionic compounds have several practical applications:
-
Refractory Materials: Many ionic compounds are used as refractory materials, capable of withstanding extremely high temperatures without melting or decomposing. This property is exploited in the construction of furnaces, kilns, and other high-temperature applications.
-
Heat Storage: The ability of ionic compounds to absorb and release large amounts of heat during melting and boiling makes them suitable for thermal energy storage systems.
Hardness and Brittleness: A Rigid Structure with a Weakness
Ionic compounds are typically hard but brittle. Their hardness arises from the strong electrostatic forces holding the ions in a rigid, three-dimensional lattice structure. It takes a considerable amount of force to break this lattice.
The Nature of Brittleness
However, the same rigid structure also accounts for their brittleness. When subjected to stress or impact, the layers of ions in the crystal lattice can shift, bringing ions of the same charge into close proximity. This results in strong electrostatic repulsion, causing the crystal to fracture along a plane. Unlike metals, which can deform plastically due to the mobile nature of their electrons, ionic compounds lack this ability, leading to their brittle behavior.
Understanding the Contrast: Hardness vs. Brittleness
It's important to differentiate hardness and brittleness. Hardness refers to resistance to scratching or indentation, while brittleness refers to the tendency to fracture under stress. Ionic compounds possess both these properties simultaneously, a consequence of their unique structure and bonding.
Practical Applications of Hardness and Brittleness
The hardness and brittleness of ionic compounds have implications in various areas:
-
Abrasives: Some ionic compounds, like silicon carbide (SiC), are exceptionally hard and used as abrasives in grinding and polishing materials.
-
Structural Materials (with caution): While brittleness limits their use in applications requiring flexibility and impact resistance, their hardness makes them suitable for certain structural applications where compressive strength is crucial and impact is minimal.
Electrical Conductivity: The Role of Ions and Their Mobility
Ionic compounds are generally poor conductors of electricity in the solid state but good conductors when molten or dissolved in water. This behavior relates directly to the mobility of the ions.
Solid State: Immobile Ions
In the solid state, the ions are held rigidly in place within the crystal lattice. They lack the freedom to move and carry an electric charge, thus resulting in poor electrical conductivity.
Molten State and Aqueous Solutions: Mobile Ions
However, when an ionic compound is melted, the ions are freed from their fixed positions and become mobile. This allows them to move under the influence of an electric field, carrying charge and enabling electrical conductivity. Similarly, when an ionic compound dissolves in water, the ions dissociate and become hydrated, meaning surrounded by water molecules. These hydrated ions are free to move through the solution, contributing to its electrical conductivity.
Electrolysis: Harnessing Conductivity
The electrical conductivity of molten ionic compounds and their aqueous solutions is exploited in a process called electrolysis. Electrolysis uses an electric current to drive chemical reactions, breaking down compounds into their constituent elements or ions. This process is widely used in various industrial applications, such as the production of metals, such as Aluminum and Sodium, and the purification of water.
Factors Affecting Conductivity
Several factors influence the electrical conductivity of molten ionic compounds and their aqueous solutions:
-
Temperature: Higher temperatures increase ion mobility, leading to improved conductivity.
-
Concentration: The concentration of ions in a solution affects the number of charge carriers available, directly impacting conductivity.
-
Nature of the solvent: The ability of the solvent to dissociate the ionic compound and solvate the ions influences the overall conductivity.
Practical Applications of Electrical Conductivity
The conductivity of molten and dissolved ionic compounds has extensive practical uses:
-
Electroplating: The deposition of a thin layer of metal onto a surface, often using an electrolyte solution containing metal ions.
-
Batteries: Many batteries rely on the movement of ions in an electrolyte solution to generate electricity.
-
Corrosion Prevention: Electrical conductivity plays a significant role in corrosion processes, and understanding this aspect helps in the development of corrosion prevention strategies.
Conclusion: Understanding the Interplay of Properties
The three physical properties discussed—high melting and boiling points, hardness and brittleness, and electrical conductivity—are intrinsically linked to the strong electrostatic forces and the rigid crystal lattice structure characteristic of ionic compounds. Understanding these properties is essential for appreciating the diverse applications of ionic compounds in various fields, highlighting their importance in materials science, chemistry, and beyond. Further exploration into the nuances of ionic bonding and crystal structures will continue to reveal new insights into these fascinating materials and their capabilities.
Latest Posts
Latest Posts
-
What Is 3 9 As A Percent
Mar 23, 2025
-
Lcm Of 8 And 12 And 15
Mar 23, 2025
-
The Circumcenter Of A Triangle Is Equidistant From The
Mar 23, 2025
-
How Are Photosynthesis And Cellular Respiration Interdependent
Mar 23, 2025
-
What Is The Percent Of 2 5
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Three Physical Properties Of Ionic Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
