Three Main Parts Of A Nucleotide
Juapaving
Mar 19, 2025 · 7 min read
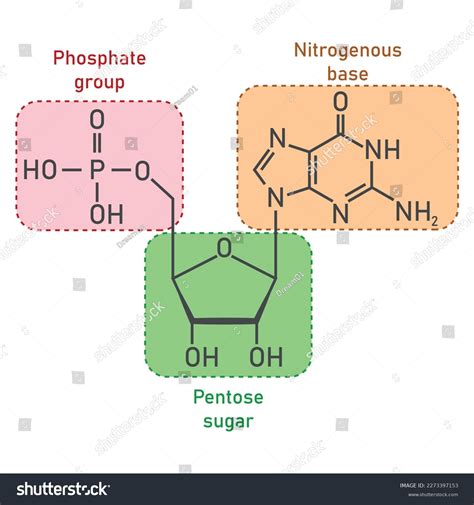
Table of Contents
Decoding the Nucleotide: A Deep Dive into its Three Main Components
Nucleotides, the fundamental building blocks of DNA and RNA, are complex molecules with crucial roles in numerous biological processes. Understanding their structure is key to understanding how genetic information is stored, replicated, and expressed. This article will explore the three main components of a nucleotide: the nitrogenous base, the pentose sugar, and the phosphate group. We'll examine each component in detail, highlighting their individual properties and how they contribute to the overall function of the nucleotide.
1. The Nitrogenous Base: The Information Carrier
The nitrogenous base forms the heart of the nucleotide, carrying the genetic information that distinguishes one nucleotide from another. These bases are organic molecules containing nitrogen atoms, and they are categorized into two main groups: purines and pyrimidines.
1.1 Purines: Adenine (A) and Guanine (G)
Purines are characterized by their double-ring structure, consisting of a six-membered ring fused to a five-membered ring. The two most common purines found in DNA and RNA are adenine (A) and guanine (G).
-
Adenine (A): Adenine is a vital component of both DNA and RNA. It pairs with thymine (T) in DNA and uracil (U) in RNA through hydrogen bonds, forming a crucial element of the double helix structure in DNA and the single-stranded structure in RNA. Adenine's role extends beyond its function in nucleic acids; it also plays a role in energy metabolism as part of adenosine triphosphate (ATP), the primary energy currency of cells. The specific arrangement of its atoms allows for the formation of stable hydrogen bonds, critical for maintaining the integrity of the DNA double helix.
-
Guanine (G): Guanine, another purine base, also contributes to the genetic code in both DNA and RNA. It pairs with cytosine (C) through three hydrogen bonds, forming a stronger bond compared to the adenine-thymine/uracil pair. This stronger bond contributes to the stability of the DNA double helix, particularly in regions where high stability is required. Guanine, like adenine, participates in various cellular processes beyond DNA and RNA synthesis.
1.2 Pyrimidines: Cytosine (C), Thymine (T), and Uracil (U)
Pyrimidines are distinguished by their single-ring structure, a six-membered ring containing nitrogen atoms. The three primary pyrimidines are cytosine (C), thymine (T), and uracil (U).
-
Cytosine (C): Cytosine is a crucial component of both DNA and RNA. It pairs with guanine (G) through three hydrogen bonds, contributing to the structural integrity of the nucleic acid molecules. Its presence in both DNA and RNA highlights its essential role in encoding genetic information. The precise arrangement of its functional groups facilitates the formation of strong hydrogen bonds with guanine.
-
Thymine (T): Thymine is unique to DNA, where it pairs with adenine (A) through two hydrogen bonds. Its presence is directly linked to the stability and replication of the DNA molecule. The methyl group on thymine is a key differentiator from uracil, and it potentially contributes to the enhanced stability of DNA compared to RNA.
-
Uracil (U): Uracil replaces thymine in RNA and pairs with adenine (A) through two hydrogen bonds. The absence of a methyl group differentiates it from thymine. Its presence in RNA is crucial for its diverse functions in protein synthesis and gene regulation. The lack of the methyl group in uracil may contribute to the increased susceptibility of RNA to degradation compared to DNA.
The specific sequence of these nitrogenous bases along the nucleotide chain determines the genetic code, essentially providing the blueprint for the synthesis of proteins and the regulation of cellular processes. The subtle differences in their structure—the presence or absence of a methyl group, the number of rings, and the arrangement of nitrogen and carbon atoms—lead to significant differences in their pairing capabilities and ultimately, the genetic information they encode.
2. The Pentose Sugar: The Structural Backbone
The second crucial component of a nucleotide is the pentose sugar, a five-carbon sugar molecule. In DNA, this sugar is deoxyribose, while in RNA, it is ribose. This seemingly minor difference has significant implications for the structure and function of each nucleic acid.
2.1 Deoxyribose in DNA: Stability and Durability
Deoxyribose, found in DNA, lacks a hydroxyl (-OH) group at the 2' carbon atom compared to ribose. This seemingly small difference significantly impacts DNA's stability. The absence of the 2'-hydroxyl group makes the DNA backbone less reactive and more resistant to hydrolysis (breakdown by water), contributing to its greater stability and suitability for long-term storage of genetic information. The stronger and more stable structure of DNA is essential for preserving the integrity of the genetic code across generations.
2.2 Ribose in RNA: Versatility and Reactivity
Ribose, present in RNA, contains a hydroxyl (-OH) group at the 2' carbon atom. This hydroxyl group makes ribose more reactive than deoxyribose. This increased reactivity contributes to RNA's greater versatility but also makes it less stable than DNA. The presence of the 2'-hydroxyl group can lead to spontaneous hydrolysis, potentially leading to degradation of the RNA molecule. However, this reactivity is also crucial for RNA's diverse catalytic and regulatory roles. RNA molecules can fold into complex three-dimensional structures that enable them to act as enzymes (ribozymes) and regulate gene expression. The inherent instability of RNA may also be advantageous in some contexts, such as the relatively short lifespan of many mRNA molecules which regulates gene expression efficiently.
The differences between deoxyribose and ribose directly impact the overall stability and functionality of DNA and RNA, reflecting the distinct roles each nucleic acid plays in the cell. The structural differences dictate their individual characteristics and impact the evolutionary pressures that have shaped their respective functions.
3. The Phosphate Group: Linking the Units and Providing Energy
The phosphate group is the third essential component of a nucleotide. It consists of a phosphorus atom bonded to four oxygen atoms, forming a negatively charged molecule. The phosphate group plays several crucial roles:
3.1 Linking Nucleotides Together: The Phosphodiester Bond
The phosphate group serves as a bridge, connecting the 3' carbon atom of one pentose sugar to the 5' carbon atom of the next sugar molecule. This linkage, known as a phosphodiester bond, forms the sugar-phosphate backbone of DNA and RNA. The negatively charged phosphate groups contribute to the overall negative charge of DNA and RNA molecules, influencing their interaction with proteins and other cellular components. The phosphodiester bond is strong and stable, ensuring the integrity of the nucleotide chain.
3.2 Energy Transfer: ATP and Other Nucleotide Triphosphates
Besides its structural role, the phosphate group also plays a significant role in energy transfer. Nucleotide triphosphates, such as adenosine triphosphate (ATP), guanosine triphosphate (GTP), and others, carry high-energy phosphate bonds. The hydrolysis (breakdown) of these bonds releases a significant amount of energy, which fuels many cellular processes, including muscle contraction, protein synthesis, and active transport across cell membranes. The energy stored in these high-energy phosphate bonds is readily available for various cellular processes. The strategic position of the phosphate groups allows for the efficient transfer and utilization of energy within the cell.
3.3 Regulation of Cellular Processes
Phosphate groups can also participate in the regulation of cellular processes through phosphorylation, a process where a phosphate group is added to a protein or other molecule. This modification can alter the activity of the target molecule, influencing various cellular pathways. The addition of a phosphate group can act as a molecular switch, activating or inhibiting the target protein, illustrating the importance of the phosphate group beyond its structural contribution. Phosphorylation is involved in a vast array of cellular signaling cascades and regulatory mechanisms.
In conclusion, the phosphate group's role extends beyond merely linking nucleotides together. It's crucial for energy storage and transfer and serves as a key regulator of cellular activities. Its negative charge also contributes to the overall properties of DNA and RNA.
Conclusion: The Interplay of Components
The three components of a nucleotide—the nitrogenous base, the pentose sugar, and the phosphate group—work together in a coordinated fashion. The nitrogenous base encodes the genetic information, the sugar forms the structural backbone, and the phosphate group links the nucleotides and plays a role in energy transfer and regulation. The subtle differences in the chemical structures of these components, particularly the sugar and the specific bases, define the unique properties of DNA and RNA, making them ideal for their respective roles in storing, replicating, and expressing genetic information. Understanding the interplay between these components is crucial for comprehending the fundamental processes of life. Future research into nucleotide chemistry continues to unveil new insights into their diverse roles and potential applications in biotechnology and medicine.
Latest Posts
Latest Posts
-
What Type Of Bond Is Kcl
Mar 19, 2025
-
Nucleic Acids Are Polymers Of Blank
Mar 19, 2025
-
Ias Exam Qualification And Age Limit
Mar 19, 2025
-
Simplify The Square Root Of 243
Mar 19, 2025
-
What Is The Net Gain Of Atp During Glycolysis
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Three Main Parts Of A Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
