Electronic Configuration Of Cr And Cu
Juapaving
Mar 17, 2025 · 5 min read
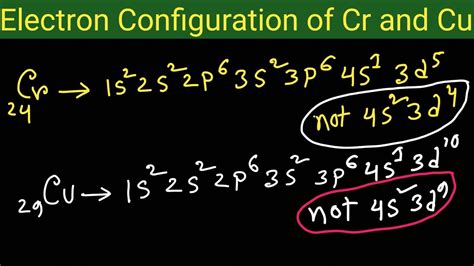
Table of Contents
The Exceptional Electronic Configurations of Chromium (Cr) and Copper (Cu): A Deep Dive
The electronic configuration of elements, a fundamental concept in chemistry, dictates their chemical and physical properties. While most elements follow Hund's rule and the Aufbau principle predictably, some exceptions exist. Chromium (Cr) and copper (Cu) are prominent examples, showcasing fascinating deviations from the expected electronic configurations. Understanding these exceptions requires delving into the intricacies of atomic orbitals and energy levels. This article will provide a comprehensive exploration of the electronic configurations of Cr and Cu, explaining the reasons behind their anomalous behavior and its implications.
Understanding Basic Electronic Configuration Principles
Before diving into the exceptions, let's establish a foundational understanding of how electronic configurations are typically determined.
The Aufbau Principle
The Aufbau principle states that electrons fill atomic orbitals in order of increasing energy levels. This generally follows a specific sequence: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, and so on.
Hund's Rule
Hund's rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This maximizes electron spin and overall stability.
Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). This means each orbital can hold a maximum of two electrons with opposite spins.
The Expected vs. Observed Electronic Configurations
Based on the Aufbau principle and Hund's rule, we would expect the electronic configuration of chromium (atomic number 24) to be: 1s²2s²2p⁶3s²3p⁶4s²3d⁴. Similarly, for copper (atomic number 29), we'd expect: 1s²2s²2p⁶3s²3p⁶4s²3d⁹.
However, experimental evidence reveals that the actual electronic configurations are:
- Chromium (Cr): 1s²2s²2p⁶3s²3p⁶4s¹3d⁵
- Copper (Cu): 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰
These deviations from the expected configurations are significant and require further explanation.
The Explanation: Stability and Exchange Energy
The key to understanding the anomalous configurations of Cr and Cu lies in the concept of exchange energy. Exchange energy is a quantum mechanical phenomenon that arises from the interaction of electrons with parallel spins. Electrons with parallel spins tend to repel each other less strongly than electrons with opposite spins. This results in a lower overall energy state for the atom when electrons are distributed to maximize parallel spins.
Chromium (Cr): Half-Filled d Subshell Stability
In the case of chromium, the half-filled 3d subshell (3d⁵) and the singly occupied 4s subshell (4s¹) provide exceptional stability. The exchange energy gained by having five unpaired electrons in the 3d subshell outweighs the energy cost of promoting an electron from the 4s orbital to the 3d orbital. A half-filled or completely filled d subshell offers significant extra stability, contributing to this phenomenon. This extra stability lowers the overall energy of the atom, making the observed configuration more favorable than the expected one.
Copper (Cu): Completely Filled d Subshell Stability
Similarly, copper achieves exceptional stability by having a completely filled 3d subshell (3d¹⁰) and a singly occupied 4s subshell (4s¹). The energy gain from the enhanced stability of a fully filled d subshell outweighs the energy required to move an electron from the 4s to the 3d orbital. A completely filled d subshell represents a state of maximum exchange energy and minimal electron-electron repulsion. This makes the observed configuration more energetically favorable than the one predicted by the Aufbau principle alone.
Implications of the Exceptional Configurations
The unusual electronic configurations of chromium and copper have significant implications for their chemical properties and behavior.
Chemical Reactivity
The stability associated with half-filled and completely filled d subshells affects the reactivity of these elements. For instance, chromium exhibits a variety of oxidation states, including +2, +3, and +6, reflecting the versatility of its electron configuration. Copper, despite having a filled d subshell, still exhibits multiple oxidation states (+1 and +2) due to the relatively close energy levels of the 4s and 3d orbitals.
Magnetic Properties
The number of unpaired electrons significantly impacts magnetic properties. Chromium, with its six unpaired electrons (one in 4s and five in 3d), is paramagnetic, meaning it is attracted to a magnetic field. Copper, with its single unpaired electron in the 4s orbital, is also paramagnetic, although to a lesser extent compared to chromium.
Spectral Properties
The electronic configuration also affects the spectral properties of the elements. The transitions of electrons between different energy levels give rise to characteristic absorption and emission spectra. The unusual electron configurations of chromium and copper lead to distinct spectral features that can be used for their identification and analysis.
Conclusion: Beyond the Rules
The exceptional electronic configurations of chromium and copper demonstrate that while the Aufbau principle and Hund's rule provide a valuable framework for predicting electronic configurations, they are not absolute laws. The interplay of factors such as exchange energy and the stability of half-filled and completely filled subshells can lead to deviations from these principles. Understanding these exceptions is crucial for a complete understanding of atomic structure and the chemical properties of the elements. This deeper understanding allows us to appreciate the nuances of quantum mechanics and their impact on the macroscopic world. The study of these exceptions, therefore, provides invaluable insights into the complex behavior of atoms and their interactions. Further research continues to explore the intricacies of electronic configurations and their role in various chemical and physical phenomena.
Latest Posts
Latest Posts
-
What Is The Lcm Of 5 4 And 3
Mar 17, 2025
-
Which Of The Following Is An Example Of A Tissue
Mar 17, 2025
-
Balanced Equation Of Sodium Carbonate And Hydrochloric Acid
Mar 17, 2025
-
Lowest Common Multiple Of 2 3 And 6
Mar 17, 2025
-
How Many Sides Of A Parallelogram
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Electronic Configuration Of Cr And Cu . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
