The Solubility Of Solids In Water
Juapaving
Mar 18, 2025 · 6 min read
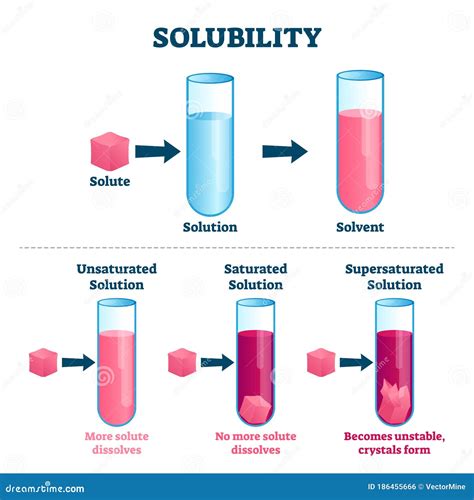
Table of Contents
The Solubility of Solids in Water: A Deep Dive
The solubility of solids in water is a fundamental concept in chemistry with far-reaching implications across various scientific disciplines and everyday life. Understanding how and why different solids dissolve in water to varying degrees is crucial for everything from designing pharmaceuticals and formulating cleaning products to interpreting geological processes and predicting environmental impacts. This comprehensive article explores the intricacies of solid solubility in water, encompassing the factors that influence it, the methods used to quantify it, and its significance in diverse fields.
What is Solubility?
Solubility refers to the maximum amount of a solute (the substance being dissolved) that can dissolve in a given amount of solvent (the substance doing the dissolving) at a specific temperature and pressure to form a saturated solution. A saturated solution is one where the solvent has dissolved the maximum amount of solute possible under those conditions. Adding more solute to a saturated solution will not result in further dissolution; instead, it will remain undissolved. Conversely, an unsaturated solution contains less solute than it can dissolve, while a supersaturated solution contains more solute than it can theoretically hold under normal conditions – a metastable state.
Key Terms to Understand:
- Solute: The substance that dissolves in a solvent. In the context of solids dissolving in water, the solid is the solute.
- Solvent: The substance that dissolves the solute. In this case, water is the solvent.
- Solution: A homogeneous mixture of a solute and a solvent.
- Saturated Solution: A solution containing the maximum amount of dissolved solute at a given temperature and pressure.
- Unsaturated Solution: A solution containing less solute than it can dissolve at a given temperature and pressure.
- Supersaturated Solution: A solution containing more solute than it can theoretically hold at a given temperature and pressure; this is a metastable state.
- Solubility Product Constant (Ksp): A measure of the solubility of a sparingly soluble ionic compound. It represents the equilibrium constant for the dissolution of the compound in water.
Factors Affecting the Solubility of Solids in Water
Several factors significantly influence the solubility of solids in water. These include:
1. Temperature:
The effect of temperature on solubility varies depending on the nature of the solute and the solvent. For most solid solutes, solubility increases with increasing temperature. This is because the increased kinetic energy of the water molecules at higher temperatures overcomes the attractive forces holding the solid's particles together, facilitating dissolution. However, there are exceptions, particularly for certain gases and a few solids whose solubility decreases with increasing temperature.
2. Pressure:
Pressure has a negligible effect on the solubility of solid solutes in liquid solvents. This is because solids are essentially incompressible, and the volume change upon dissolution is minimal. The effect of pressure is far more pronounced on the solubility of gases in liquids.
3. Nature of the Solute and Solvent:
The "like dissolves like" principle is a crucial concept. Polar solvents, such as water, tend to dissolve polar solutes (those with unevenly distributed charges), while nonpolar solvents dissolve nonpolar solutes. Ionic compounds (composed of charged ions) are often readily soluble in water because the polar water molecules can interact strongly with the ions, surrounding and stabilizing them. Conversely, nonpolar solids, like many hydrocarbons, are generally insoluble in water.
4. Particle Size:
Smaller particles of a solid generally dissolve faster than larger particles. This is because smaller particles have a larger surface area to volume ratio, making more of the solid accessible to the solvent molecules. However, particle size does not alter the ultimate solubility; it only affects the rate of dissolution.
5. Presence of Other Substances:
The presence of other substances in the solution can significantly affect solubility. Common ion effect: The solubility of a sparingly soluble ionic compound decreases when a soluble salt containing a common ion is added to the solution. This is due to Le Chatelier's principle, which states that the equilibrium will shift to counteract the added stress. Complex ion formation: Certain ions can form complexes with the solute, increasing its solubility.
Measuring Solubility
Solubility is typically expressed as the concentration of the saturated solution at a given temperature. Common units include:
- Grams per liter (g/L): The mass of solute (in grams) dissolved in one liter of solvent.
- Moles per liter (mol/L) or molarity (M): The amount of solute (in moles) dissolved in one liter of solvent.
- Parts per million (ppm) or parts per billion (ppb): Used to express the solubility of very sparingly soluble substances.
The experimental determination of solubility often involves:
- Preparation of a saturated solution: A known amount of solute is added to a known amount of solvent, and the mixture is stirred until no more solute dissolves.
- Separation of undissolved solute: The saturated solution is filtered to remove any undissolved solute.
- Analysis of the solution: The concentration of the solute in the saturated solution is determined using appropriate analytical techniques, such as titration or spectroscopy.
Applications and Significance of Solid Solubility in Water
The solubility of solids in water plays a crucial role in diverse fields:
1. Pharmaceuticals:
Solubility is a critical factor in drug development and delivery. Drugs need to be sufficiently soluble in water or bodily fluids to be absorbed and reach their target sites effectively. Poorly soluble drugs often require formulation strategies to enhance their solubility and bioavailability.
2. Environmental Science:
Understanding the solubility of various substances in water is crucial for assessing environmental risks. The solubility of pollutants determines their mobility in soil and water, impacting their potential to contaminate water sources and harm ecosystems.
3. Geology:
The solubility of minerals in water governs the formation and weathering of rocks and sediments. The dissolution and precipitation of minerals play essential roles in geological processes, including cave formation and the formation of mineral deposits.
4. Agriculture:
The solubility of fertilizers determines their availability to plants. Water-soluble fertilizers provide readily available nutrients, while less soluble fertilizers release nutrients more slowly.
5. Food Science:
The solubility of various food components affects their texture, taste, and stability. For example, the solubility of sugars and salts influences the sweetness and saltiness of foods.
6. Industrial Processes:
Many industrial processes rely on the solubility of various substances in water. Examples include the production of chemicals, the treatment of wastewater, and the cleaning of industrial equipment.
Solubility and the Ksp: Sparingly Soluble Salts
For sparingly soluble ionic compounds (salts), solubility is often expressed using the solubility product constant, Ksp. Ksp is the equilibrium constant for the dissolution reaction of the salt in water. A smaller Ksp value indicates lower solubility. The Ksp value can be used to calculate the molar solubility of the salt, which represents the concentration of the metal cation in a saturated solution.
Understanding Ksp allows for the prediction of precipitation reactions. If the ionic product (the product of the concentrations of the ions raised to their stoichiometric coefficients) exceeds the Ksp, precipitation will occur.
Conclusion
The solubility of solids in water is a multifaceted phenomenon with significant implications across numerous disciplines. Understanding the factors that influence solubility, the methods for measuring it, and its practical applications is fundamental for advancements in various fields, from medicine and environmental science to geology and industrial processes. This knowledge is essential for developing new technologies, addressing environmental challenges, and improving our understanding of the natural world. The concepts discussed herein provide a solid foundation for further exploration of this crucial area of chemistry.
Latest Posts
Latest Posts
-
How Many Electrons Can 3d Hold
Mar 18, 2025
-
Group 18 Elements Were Called The Noble Gases Originally Because
Mar 18, 2025
-
What Is The Electron Configuration For Barium
Mar 18, 2025
-
What Are The Common Factors Of 28
Mar 18, 2025
-
52 Rounded To The Nearest Tenth
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Solubility Of Solids In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
