The Most Abundant Gas In The Atmosphere
Juapaving
Apr 06, 2025 · 6 min read
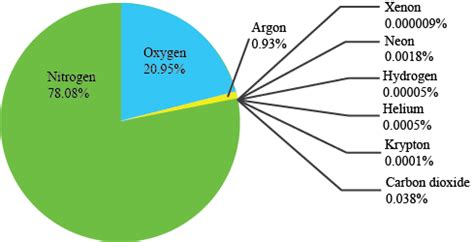
Table of Contents
The Most Abundant Gas in the Atmosphere: A Deep Dive into Nitrogen
Nitrogen, the unsung hero of our atmosphere, makes up a whopping 78% of the air we breathe. While oxygen often steals the spotlight for its crucial role in respiration, nitrogen plays a surprisingly diverse and vital role in sustaining life on Earth. This article delves deep into the properties, importance, and fascinating aspects of this ubiquitous gas.
Understanding Nitrogen: Properties and Characteristics
Nitrogen (N₂), a colorless, odorless, and tasteless diatomic gas, is a crucial element found throughout the universe. Its unique properties stem from the strong triple bond connecting its two nitrogen atoms. This triple bond requires significant energy to break, rendering nitrogen relatively inert under standard conditions. This inertness is both a blessing and a curse – it makes nitrogen a stable atmospheric component but also limits its direct availability to living organisms.
Key Properties of Nitrogen:
- Chemical Symbol: N
- Atomic Number: 7
- Atomic Weight: 14.007
- Melting Point: -210°C (-346°F)
- Boiling Point: -196°C (-321°F)
- Density: 1.25 g/L (at standard temperature and pressure)
- Oxidation States: -3, -2, -1, 0, +1, +2, +3, +4, +5
This seemingly simple molecule possesses a surprisingly complex chemistry, particularly under specialized conditions where the strong triple bond can be broken or modified. This ability to participate in chemical reactions, albeit under specific circumstances, makes nitrogen a cornerstone of countless processes vital to life on Earth.
The Role of Nitrogen in the Atmosphere: More Than Just Filler
While often overlooked in favor of oxygen, nitrogen's presence in the atmosphere is far from passive. Its inert nature prevents it from readily reacting with other atmospheric components, thus maintaining the stability of our atmosphere. This stability is crucial for the protection of life from harmful radiation and temperature fluctuations. Furthermore, nitrogen plays a dynamic role in several key atmospheric processes:
1. Maintaining Atmospheric Pressure:
The sheer abundance of nitrogen contributes significantly to the Earth's atmospheric pressure, a vital factor influencing weather patterns and protecting us from the vacuum of space. Without this substantial pressure, liquid water would not exist on Earth's surface, making life as we know it impossible.
2. Moderating Temperature Fluctuations:
Nitrogen's high heat capacity helps moderate temperature fluctuations, preventing extreme temperature swings between day and night. This temperature buffering is critical for maintaining habitable conditions on Earth.
3. Shielding from Harmful Radiation:
Although not a primary component of the ozone layer, nitrogen molecules help absorb some ultraviolet radiation from the sun, contributing to Earth's overall protection from harmful radiation.
The Nitrogen Cycle: A Continuous Process of Transformation
Nitrogen's inertness is a double-edged sword. While providing atmospheric stability, it makes nitrogen largely unavailable to living organisms in its diatomic form (N₂). This is where the nitrogen cycle comes into play – a crucial biogeochemical cycle that converts nitrogen into usable forms for plants and animals.
Stages of the Nitrogen Cycle:
-
Nitrogen Fixation: Specialized bacteria, both free-living and symbiotic with plants (like legumes), convert atmospheric nitrogen (N₂) into ammonia (NH₃) or ammonium (NH₄⁺), forms usable by plants. This is a crucial step because it's the only way atmospheric nitrogen is made biologically available.
-
Nitrification: Ammonia is further oxidized by other bacteria into nitrites (NO₂⁻) and then nitrates (NO₃⁻), more readily absorbed by plants. This process releases energy used by nitrifying bacteria.
-
Assimilation: Plants absorb nitrates and ammonium through their roots and incorporate nitrogen into their tissues, building proteins, nucleic acids (DNA and RNA), and other essential biomolecules. Animals obtain nitrogen by consuming plants or other animals.
-
Ammonification: When plants and animals die or excrete waste, decomposers (bacteria and fungi) break down organic nitrogen compounds back into ammonia.
-
Denitrification: Certain bacteria convert nitrates back into atmospheric nitrogen (N₂), completing the cycle. This process occurs in oxygen-poor environments, like waterlogged soils.
This intricate cycle ensures the continuous flow of nitrogen through the biosphere, demonstrating the critical role of nitrogen in maintaining life's delicate balance.
Industrial Applications of Nitrogen: From Food Preservation to Steel Production
Nitrogen's inertness is not just a natural phenomenon; it's also exploited extensively in numerous industrial applications:
1. Food Preservation:
Nitrogen's inert nature makes it an ideal packaging gas for extending the shelf life of food products. By replacing oxygen in packaging, nitrogen prevents oxidation and the growth of microorganisms, reducing spoilage and preserving freshness. This is widely used in packaging chips, coffee, and other perishable goods.
2. Steel Production:
Nitrogen is employed in steel production to modify the steel's properties, enhancing its strength and durability. By controlling nitrogen content, manufacturers can produce steel with specific characteristics suitable for various applications.
3. Chemical Industry:
Nitrogen serves as a crucial raw material in the production of ammonia, a key ingredient in fertilizers. Ammonia production via the Haber-Bosch process is one of the most important industrial processes, providing essential nitrogen for agriculture.
4. Electronics Manufacturing:
Nitrogen's inertness is also beneficial in electronics manufacturing, where it's used as a protective atmosphere during sensitive processes to prevent oxidation and contamination.
5. Cryogenics:
Liquid nitrogen, obtained by cooling gaseous nitrogen, is widely used in cryogenics, enabling extremely low temperatures for various applications, including medical treatments, cryopreservation, and scientific research.
The Environmental Impact of Nitrogen: A Double-Edged Sword
While nitrogen is essential for life, human activities have significantly altered the nitrogen cycle, leading to several environmental concerns:
1. Eutrophication:
Excessive nitrogen from fertilizers and industrial emissions can lead to eutrophication in aquatic systems. Excess nitrogen fuels algal blooms, depleting oxygen levels and harming aquatic life. This can result in “dead zones” – areas with little or no oxygen, incapable of supporting life.
2. Acid Rain:
Nitrogen oxides (NOx) released from combustion processes can contribute to acid rain, harming ecosystems and infrastructure. Acid rain damages forests, acidifies lakes and rivers, and corrodes buildings and monuments.
3. Greenhouse Gas Effects:
Nitrous oxide (N₂O), a potent greenhouse gas, is released from agricultural activities and other sources, contributing to climate change. N₂O is a significantly more potent greenhouse gas than carbon dioxide.
4. Ozone Depletion:
Although less significant than the impact of chlorofluorocarbons (CFCs), nitrogen oxides can contribute to ozone depletion in the stratosphere, reducing Earth's protection from harmful ultraviolet radiation.
The Future of Nitrogen Research and Management
Understanding and managing nitrogen's role in the environment is crucial for maintaining a sustainable future. Continued research is focused on:
-
Developing more efficient nitrogen fertilizers: Reducing nitrogen loss from fertilizers is critical to minimizing eutrophication and greenhouse gas emissions.
-
Improving nitrogen fixation techniques: Optimizing nitrogen fixation by legumes and other methods can enhance crop yields while reducing the need for synthetic fertilizers.
-
Developing innovative technologies for NOx reduction: Reducing NOx emissions from industrial processes and vehicles is essential for mitigating acid rain and ozone depletion.
-
Monitoring and modeling the nitrogen cycle: Advanced monitoring and modeling techniques are essential for understanding the complex interactions within the nitrogen cycle and predicting the effects of human activities.
Conclusion: Appreciating the Ubiquitous and Essential Nitrogen
Nitrogen, though often overlooked, is a fundamental component of our atmosphere and a crucial element for life on Earth. Its inert nature provides atmospheric stability, while its ability to participate in the nitrogen cycle makes it an essential building block of life. Understanding its properties, its role in the environment, and its industrial applications is crucial for managing its impact and ensuring a sustainable future. From the air we breathe to the food we eat, nitrogen's influence is pervasive and undeniable, highlighting the importance of continued research and responsible management of this vital element. The seemingly simple nitrogen molecule is a testament to the intricate complexity and interconnectedness of life on our planet.
Latest Posts
Latest Posts
-
Is 1 Chloro 2 Methylpentane Chiral
Apr 07, 2025
-
In What Organelle Does Photosynthesis Take Place
Apr 07, 2025
-
Ecological Pyramids Are One Way To Demonstrate
Apr 07, 2025
-
What Is The Multiple Of 17
Apr 07, 2025
-
Is Heat Conductivity A Chemical Change
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about The Most Abundant Gas In The Atmosphere . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
