Is Heat Conductivity A Chemical Change
Juapaving
Apr 07, 2025 · 5 min read
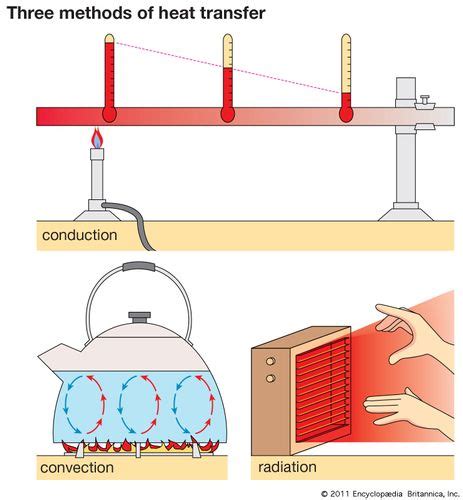
Table of Contents
Is Heat Conductivity a Chemical Change? Understanding the Difference Between Physical and Chemical Properties
Heat conductivity, a property describing a material's ability to transfer heat, is often confused with chemical changes. However, heat conductivity is fundamentally a physical property, not a chemical one. This article will delve deep into the distinction between physical and chemical changes, thoroughly examining heat conductivity and its role within the broader context of material properties. We will explore various examples and address common misconceptions to provide a comprehensive understanding of this important concept.
Understanding Physical and Chemical Changes
Before we delve into the specifics of heat conductivity, it's crucial to establish a clear understanding of the difference between physical and chemical changes. This distinction is fundamental to categorizing properties like heat conductivity.
Physical Changes
Physical changes alter the form or appearance of a substance without changing its chemical composition. These changes are often reversible. Examples include:
- Changes in state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid), and deposition (gas to solid). These transformations affect the arrangement of molecules but not the molecules themselves.
- Shape changes: Cutting paper, bending a metal rod, or crushing a can alters the physical form but not the chemical makeup.
- Dissolving: Salt dissolving in water is a physical change; the salt molecules are dispersed but remain chemically unchanged. Evaporation of the water recovers the original salt.
- Mixing: Mixing sand and water creates a mixture, but neither substance undergoes a chemical transformation.
Chemical Changes
Chemical changes, also known as chemical reactions, result in the formation of new substances with different chemical properties and compositions. These changes are often irreversible. Examples include:
- Burning: Combustion reactions like burning wood involve the breaking and formation of chemical bonds, producing new substances like carbon dioxide and water.
- Rusting: The oxidation of iron, forming iron oxide (rust), is a chemical change.
- Cooking: Cooking an egg involves irreversible chemical changes in the egg proteins.
- Digestion: The breakdown of food in our bodies involves numerous chemical reactions.
Heat Conductivity: A Physical Property
Heat conductivity, or thermal conductivity, refers to a material's ability to transfer heat energy from one region to another. It's a measure of how efficiently heat flows through a substance. Materials with high thermal conductivity, like metals, transfer heat quickly, while those with low thermal conductivity, like insulators (wood, plastic, etc.), transfer heat slowly.
The mechanism of heat transfer in materials is primarily related to the movement of particles (atoms, molecules, or electrons). In metals, free electrons play a crucial role in rapid heat conduction. In non-metals, heat is primarily transferred through vibrations of atoms and molecules within the material's structure.
Importantly, the process of heat transfer does not alter the chemical composition of the material. The material remains the same substance before and after heat is transferred through it. This is the key reason why heat conductivity is considered a physical property.
Examples illustrating Heat Conductivity as a Physical Change
Let's consider a few examples to solidify the understanding:
- Heating a copper pan: When you heat a copper pan on a stove, the heat energy is rapidly transferred through the copper due to its high thermal conductivity. The copper itself doesn't undergo a chemical change; it remains copper. The increased temperature is a physical change, not a chemical one.
- Insulating a house: Houses are insulated to reduce heat transfer. Insulating materials like fiberglass or foam have low thermal conductivity, thus slowing down the rate of heat transfer. The insulation material's chemical composition is unaffected by the heat flow.
- Boiling water in a metal pot: The metal pot efficiently transfers heat to the water, causing it to boil. The chemical composition of both the water and the metal pot remain unchanged during the heating process. The boiling of the water itself is a physical change (liquid to gas).
Addressing Common Misconceptions
Several misconceptions surround heat conductivity and chemical changes. Let's address some common ones:
- Heat causing chemical decomposition: While high temperatures can certainly initiate chemical reactions, such as decomposition, the heat conductivity itself is not the cause of the chemical change. The heat simply provides the energy needed for the reaction to occur. The material's ability to conduct that heat (its thermal conductivity) is separate from the chemical reaction it might trigger.
- Color change due to heating: Some materials change color when heated, which might seem like a chemical change. However, many times, this color change is a result of a reversible physical change (like a change in crystal structure) rather than an irreversible chemical reaction. The original color may return upon cooling.
- Phase changes and chemical changes: It's crucial to remember that phase changes (solid, liquid, gas) are physical changes, not chemical changes. Even though heat is involved in these phase transitions, the chemical composition remains constant. The heat conductivity of the material influences the rate of the phase change, but not the nature of the change itself.
Factors Affecting Heat Conductivity
Several factors influence a material's heat conductivity:
- Temperature: Thermal conductivity often varies with temperature. For most materials, it decreases with increasing temperature.
- Density: Denser materials generally have higher thermal conductivity.
- Crystal structure: The arrangement of atoms and molecules in a material significantly influences its thermal conductivity. Crystalline materials generally have higher thermal conductivity than amorphous materials.
- Presence of impurities: Impurities in a material can affect its thermal conductivity.
- Phase of matter: The phase of a substance (solid, liquid, gas) significantly impacts its thermal conductivity. Solids typically have the highest thermal conductivity, followed by liquids, and then gases.
Conclusion: Heat Conductivity Remains a Physical Property
In conclusion, heat conductivity is definitively a physical property, not a chemical one. The process of heat transfer does not alter the chemical composition of the material. While heat can trigger chemical reactions or physical changes like phase transitions, the material's ability to conduct that heat is separate from the resulting chemical or physical transformations. Understanding this distinction is crucial for comprehending material behavior and designing materials with specific properties for various applications. By clarifying the difference between physical and chemical properties and the role of heat conductivity, we enhance our understanding of the fundamental principles governing the behavior of matter. This knowledge is essential in diverse fields, including engineering, material science, and thermal physics.
Latest Posts
Latest Posts
-
Reaction Of Sodium Hydroxide And Calcium Chloride
Apr 10, 2025
-
High Government Expenditures Can Lead To A Bigger
Apr 10, 2025
-
Area Of A Circle With Radius 5
Apr 10, 2025
-
32 Degrees Is What In Celsius
Apr 10, 2025
-
3 Inches Is How Many Centimeters
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about Is Heat Conductivity A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.