The Molar Mass Of Cuso4.5h2o Is 249
Juapaving
Mar 18, 2025 · 5 min read
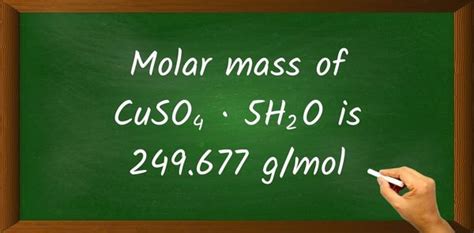
Table of Contents
The Molar Mass of CuSO₄·5H₂O is 249: A Deep Dive into Copper(II) Sulfate Pentahydrate
The statement "the molar mass of CuSO₄·5H₂O is 249" is a fundamental fact in chemistry, specifically concerning the compound copper(II) sulfate pentahydrate. This seemingly simple statement, however, opens the door to a wealth of understanding about chemical formulas, molar mass calculations, and the properties of this fascinating compound. This article will explore this statement in detail, delving into the calculation itself, the significance of the components, and the broader applications of copper(II) sulfate pentahydrate.
Understanding the Chemical Formula: CuSO₄·5H₂O
Before we dive into the molar mass calculation, it's crucial to understand what the chemical formula CuSO₄·5H₂O represents. This formula describes copper(II) sulfate pentahydrate, a hydrated salt. Let's break it down:
-
Cu: Represents copper, a transition metal with atomic number 29. In this compound, copper exists in its +2 oxidation state (Cu²⁺).
-
S: Represents sulfur, a non-metal with atomic number 16.
-
O: Represents oxygen, a non-metal with atomic number 8. There are four oxygen atoms bonded to the sulfur atom to form a sulfate ion (SO₄²⁻).
-
·5H₂O: This signifies the presence of five water molecules associated with each formula unit of copper(II) sulfate. These water molecules are not directly bonded to the copper or sulfur ions but are held within the crystal lattice structure through weaker interactions like hydrogen bonding. This is what makes it a hydrate. The term "pentahydrate" specifically indicates the presence of five water molecules.
Understanding this formula is the first step in accurately calculating the molar mass.
Calculating the Molar Mass: A Step-by-Step Guide
The molar mass of a compound is the mass of one mole of that compound, expressed in grams per mole (g/mol). To calculate the molar mass of CuSO₄·5H₂O, we need to consider the atomic masses of each element present and the number of atoms of each element in the formula. We'll use the standard atomic masses:
- Cu: 63.55 g/mol
- S: 32.07 g/mol
- O: 16.00 g/mol
- H: 1.01 g/mol
Here's the calculation:
-
Copper (Cu): 1 atom × 63.55 g/mol = 63.55 g/mol
-
Sulfur (S): 1 atom × 32.07 g/mol = 32.07 g/mol
-
Oxygen (O) in CuSO₄: 4 atoms × 16.00 g/mol = 64.00 g/mol
-
Oxygen (O) in 5H₂O: 5 molecules × 1 molecule/10 atoms × 10 atoms × 16.00 g/mol = 80.00 g/mol
-
Hydrogen (H) in 5H₂O: 5 molecules × 2 atoms/molecule × 1.01 g/mol = 10.10 g/mol
-
Total Molar Mass: 63.55 g/mol + 32.07 g/mol + 64.00 g/mol + 80.00 g/mol + 10.10 g/mol = 249.72 g/mol
Therefore, the molar mass of CuSO₄·5H₂O is approximately 249.72 g/mol, which is often rounded to 249 g/mol for simplification. The slight discrepancy between our calculated value and the stated 249 g/mol is due to rounding of the atomic masses.
The Significance of the Molar Mass
The molar mass of CuSO₄·5H₂O (249 g/mol) is not just a numerical value; it's a crucial piece of information for various chemical calculations and applications:
-
Stoichiometry: Molar mass is essential for stoichiometric calculations, which determine the quantitative relationships between reactants and products in chemical reactions. Knowing the molar mass allows for the conversion between mass and moles, vital for determining reaction yields and limiting reagents.
-
Solution Preparation: When preparing solutions of a specific concentration (e.g., molarity), the molar mass is used to calculate the required mass of CuSO₄·5H₂O to dissolve in a given volume of solvent to achieve the desired concentration.
-
Titrations: In titrations, where the concentration of an unknown solution is determined, the molar mass plays a vital role in calculating the concentration of the analyte.
-
Crystallography: The molar mass contributes to understanding the crystal structure and density of CuSO₄·5H₂O.
-
Other Applications: The molar mass is used in many other areas, such as determining the purity of a sample, analyzing chemical composition, and in various industrial processes involving CuSO₄·5H₂O.
Properties and Applications of Copper(II) Sulfate Pentahydrate
Copper(II) sulfate pentahydrate is a versatile compound with numerous applications, driven by its unique properties:
-
Appearance: It's a bright blue crystalline solid. This characteristic blue color is due to the presence of the hydrated copper(II) ion (Cu(H₂O)₄²⁺).
-
Solubility: It is highly soluble in water, readily dissolving to form a blue solution. The solubility varies with temperature.
-
Thermal Decomposition: Upon heating, CuSO₄·5H₂O loses its water molecules in a stepwise manner, eventually forming anhydrous copper(II) sulfate (CuSO₄), a white powder. This dehydration process is reversible.
-
Applications: CuSO₄·5H₂O has diverse applications:
- Agriculture: It's used as a fungicide and algaecide.
- Medicine: Historically used as an emetic, though this use is now less common.
- Industry: Employed in electroplating, as a mordant in dyeing, and in the production of other copper compounds.
- Education: A common compound used in chemistry demonstrations and experiments to illustrate concepts like hydration, dehydration, and stoichiometry.
Anhydrous Copper(II) Sulfate and the Importance of Hydration
It's important to differentiate between CuSO₄·5H₂O (the pentahydrate) and anhydrous CuSO₄. Anhydrous copper(II) sulfate is white, while the pentahydrate is blue. The water molecules are crucial for the blue color and certain properties. The process of hydration and dehydration is reversible, offering interesting properties for various applications. For instance, the color change during dehydration is used as an indicator of water presence.
Conclusion: The Significance of a Simple Equation
The seemingly simple statement "the molar mass of CuSO₄·5H₂O is 249" encapsulates a significant amount of chemical knowledge. Understanding this molar mass, its calculation, and the properties of copper(II) sulfate pentahydrate are fundamental for anyone studying chemistry or working with this versatile compound in any application. The knowledge gained expands into broader applications involving stoichiometry, solution preparation, and numerous industrial and scientific processes. By accurately determining the molar mass, we open the door to a deeper understanding of chemical reactions and the properties of this vital compound. This calculation serves as a cornerstone for numerous chemical calculations and provides a basis for understanding the significance of hydration in chemical compounds and their applications.
Latest Posts
Latest Posts
-
Whats The Lcm Of 12 And 18
Mar 18, 2025
-
How Many Liters Is 4 Gallons
Mar 18, 2025
-
How Many Ft Is 50 Meters
Mar 18, 2025
-
What Quadrilateral Has Exactly One Pair Of Parallel Sides
Mar 18, 2025
-
Which Class Of Molecules Is The Most Antigenic
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Molar Mass Of Cuso4.5h2o Is 249 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
