The Electrons Present In The Outermost Shell Are Called
Juapaving
Mar 28, 2025 · 6 min read
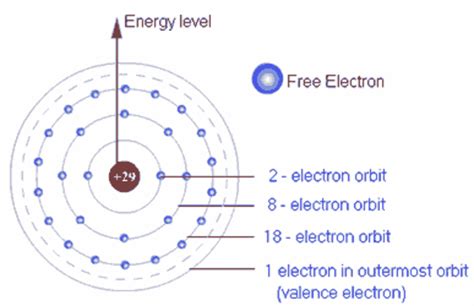
Table of Contents
The Electrons Present in the Outermost Shell are Called Valence Electrons: A Deep Dive into Atomic Structure and Chemical Bonding
The electrons present in the outermost shell of an atom are called valence electrons. These electrons are crucial in determining an element's chemical properties and how it interacts with other elements to form chemical bonds. Understanding valence electrons is fundamental to grasping the principles of chemistry, from simple ionic compounds to complex organic molecules. This comprehensive article will explore the significance of valence electrons, their role in chemical bonding, and their impact on the periodic table's organization.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell, also known as the valence shell, of an atom. They are the electrons furthest from the atom's nucleus and are therefore the least tightly bound. This loose binding makes them the most readily available for interaction with other atoms. The number of valence electrons an atom possesses significantly influences its reactivity and the types of chemical bonds it can form.
Identifying Valence Electrons: A Step-by-Step Guide
Determining the number of valence electrons for an atom can be done using the element's position on the periodic table or its electron configuration.
1. Using the Periodic Table: The periodic table is cleverly organized to reflect the electron configuration of elements. The group number (vertical column) for main group elements (Groups 1-18, excluding transition metals) directly indicates the number of valence electrons.
- Group 1 (Alkali Metals): 1 valence electron
- Group 2 (Alkaline Earth Metals): 2 valence electrons
- Group 13 (Boron Group): 3 valence electrons
- Group 14 (Carbon Group): 4 valence electrons
- Group 15 (Pnictogens): 5 valence electrons
- Group 16 (Chalcogens): 6 valence electrons
- Group 17 (Halogens): 7 valence electrons
- Group 18 (Noble Gases): 8 valence electrons (except helium, which has 2)
2. Using Electron Configuration: The electron configuration provides a detailed description of how electrons are arranged in an atom's energy levels and subshells. The valence electrons are those in the highest energy level (principal quantum number, n). For example, consider oxygen (O), which has an atomic number of 8. Its electron configuration is 1s²2s²2p⁴. The outermost shell is the second energy level (n=2), containing 2s²2p⁴ electrons, resulting in a total of 6 valence electrons.
The Significance of Valence Electrons
The number of valence electrons dictates an atom's chemical behavior. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gases with their full valence shells (octet rule, with the exception of helium). This drive for stability is the fundamental driving force behind chemical bonding.
Chemical Bonding and Valence Electrons
Atoms interact to achieve stable electron configurations through the formation of chemical bonds. Valence electrons play a central role in this process, participating directly in bond formation. The three main types of chemical bonds are:
1. Ionic Bonds: The Transfer of Electrons
Ionic bonds occur when one atom transfers one or more valence electrons to another atom. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond. A classic example is the formation of sodium chloride (NaCl), where sodium (Na) loses one valence electron to chlorine (Cl), resulting in Na⁺ and Cl⁻ ions, which are held together by ionic bonds.
2. Covalent Bonds: The Sharing of Electrons
Covalent bonds arise when atoms share valence electrons to achieve a stable electron configuration. This sharing creates a stable electron cloud between the bonded atoms. Covalent bonds are common in molecules like water (H₂O) and methane (CH₄). In water, oxygen shares electrons with two hydrogen atoms, and in methane, carbon shares electrons with four hydrogen atoms. The number of covalent bonds an atom can form is often related to the number of unpaired valence electrons it possesses.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds are characteristic of metals and involve the delocalization of valence electrons. These electrons are not associated with any specific atom but rather move freely throughout the metal lattice, forming a "sea" of electrons. This sea of electrons explains the unique properties of metals, such as their high electrical and thermal conductivity, malleability, and ductility.
Valence Electrons and the Periodic Table
The periodic table's organization is directly related to the arrangement of electrons in atoms, specifically their valence electrons. Elements in the same group (vertical column) have the same number of valence electrons, leading to similar chemical properties. This is why elements within a group often exhibit similar reactivity and form similar types of compounds.
Predicting Chemical Behavior based on Valence Electrons
Knowing the number of valence electrons allows us to predict the likely chemical behavior of an element:
- Elements with few valence electrons (1-3): Tend to lose valence electrons to form positive ions and participate in ionic bonding.
- Elements with many valence electrons (5-7): Tend to gain electrons to form negative ions and participate in ionic bonding.
- Elements with four valence electrons: Often form covalent bonds by sharing electrons.
- Elements with eight valence electrons (noble gases): Typically unreactive due to their stable electron configuration.
Beyond the Octet Rule: Exceptions and Complications
While the octet rule provides a useful guideline for predicting chemical behavior, there are exceptions. Some atoms can achieve stability with less than eight valence electrons (e.g., boron in BF₃) or more than eight valence electrons (e.g., phosphorus in PF₅). These exceptions often involve atoms from the third period (and beyond) and their ability to utilize d orbitals for bonding.
Advanced Concepts: Oxidation States and Formal Charges
The concept of valence electrons extends to more advanced topics, including oxidation states and formal charges. Oxidation states represent the apparent charge on an atom in a compound, considering the electrons involved in bonding. Formal charges help in assessing the distribution of electrons in a molecule and predicting its stability. Understanding these concepts requires a deeper understanding of electron bookkeeping and bonding theory.
Conclusion: The Importance of Valence Electrons in Chemistry
Valence electrons are the cornerstone of understanding chemical reactivity and bonding. Their number dictates an atom's tendency to form bonds and its overall chemical behavior. The periodic table's structure reflects the pattern of valence electrons, facilitating predictions about chemical properties. While the octet rule provides a helpful simplification, acknowledging exceptions and delving into more advanced concepts like oxidation states and formal charges are crucial for a comprehensive grasp of chemical principles. Mastering the concept of valence electrons unlocks a deeper understanding of the world around us, from the formation of simple salts to the complexity of biological molecules. The importance of valence electrons extends far beyond introductory chemistry, providing a solid foundation for understanding diverse areas such as materials science, biochemistry, and environmental chemistry. Understanding valence electrons is truly fundamental to appreciating the elegance and power of chemical interactions.
Latest Posts
Latest Posts
-
How Many Inches Is 2 Meters
Mar 31, 2025
-
What Medical Problem Afflicts Mrs Mallard
Mar 31, 2025
-
2 Over 5 As A Percent
Mar 31, 2025
-
35 Inches Is How Many Feet
Mar 31, 2025
-
3 Cm Is How Many Mm
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Electrons Present In The Outermost Shell Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
