The Basic Building Block Of Matter.
Juapaving
Mar 06, 2025 · 7 min read
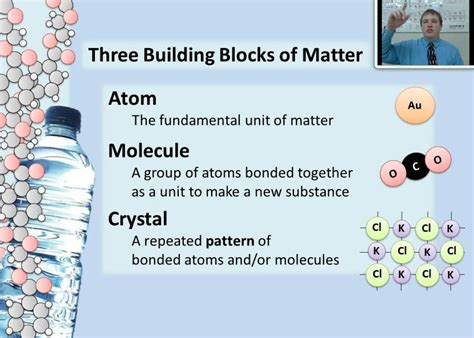
Table of Contents
The Basic Building Blocks of Matter: A Deep Dive into Atoms and Beyond
Understanding the universe starts with understanding its fundamental constituents. For centuries, philosophers and scientists alike have pondered the nature of matter, seeking the smallest, indivisible unit. While the ancient Greek concept of the "atomos," meaning "uncuttable," proved to be an oversimplification, it laid the groundwork for our modern understanding of the basic building blocks of matter: atoms, and the even smaller particles within them. This article will delve into the fascinating world of atomic structure, exploring the fundamental forces that govern their interactions and examining the implications for our understanding of the universe.
Atoms: The Indivisible (Almost)
For a long time, the atom was considered the smallest unit of matter. Defined as the smallest particle of an element that retains its chemical properties, atoms are incredibly tiny, with diameters typically measured in angstroms (1 angstrom = 0.1 nanometers). Despite their size, atoms are remarkably complex structures containing a nucleus and an electron cloud.
The Atomic Nucleus: A Dense Core
At the heart of every atom lies the nucleus, a densely packed region containing two types of particles: protons and neutrons. These particles are collectively known as nucleons.
- Protons: Positively charged particles that determine the element's atomic number and thus its identity. The number of protons defines the element; for example, all hydrogen atoms have one proton, all helium atoms have two, and so on.
- Neutrons: Neutral particles (no charge) that contribute to the atom's mass but not its chemical properties. Isotopes of an element have the same number of protons but different numbers of neutrons. For instance, carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons.
The strong nuclear force is responsible for holding the protons and neutrons together within the nucleus. This force is incredibly powerful at short distances, overcoming the electrostatic repulsion between positively charged protons. Without the strong nuclear force, atomic nuclei would immediately fly apart.
The Electron Cloud: A Realm of Probability
Surrounding the nucleus is a cloud of negatively charged electrons. These electrons are far smaller and lighter than protons and neutrons, and their movement is governed by the laws of quantum mechanics. Electrons don't orbit the nucleus in well-defined paths like planets orbiting a star. Instead, their positions are described by probability distributions, known as orbitals, which represent the regions where there is a high probability of finding an electron.
The number of electrons in a neutral atom is equal to the number of protons in the nucleus. This ensures that the atom carries no overall electric charge. However, atoms can gain or lose electrons to form ions, which are charged particles. Cations are positively charged ions (having lost electrons), while anions are negatively charged ions (having gained electrons).
The Forces That Shape the Universe
The behavior of atoms and their interactions are governed by four fundamental forces:
1. Strong Nuclear Force
As mentioned earlier, this force is responsible for binding protons and neutrons together in the nucleus. It's the strongest of the four fundamental forces but acts only over extremely short distances. Its strength explains the stability of atomic nuclei, despite the repulsive forces between protons.
2. Electromagnetic Force
This force governs the interactions between charged particles, including protons and electrons. It's responsible for the attraction between the positively charged nucleus and the negatively charged electrons, holding the atom together. Electromagnetic forces are also responsible for chemical bonding, which is crucial for the formation of molecules and the vast diversity of chemical compounds.
3. Weak Nuclear Force
This force is responsible for certain types of radioactive decay, specifically beta decay, where a neutron transforms into a proton, an electron, and an antineutrino. It is much weaker than the strong nuclear force but plays a crucial role in nuclear processes.
4. Gravitational Force
This is the weakest of the four fundamental forces, but it acts over vast distances and is responsible for the attraction between objects with mass. While gravity plays a significant role in the large-scale structure of the universe, its effects on individual atoms are negligible compared to the electromagnetic and strong nuclear forces.
Beyond Atoms: Subatomic Particles
The quest to understand the basic building blocks of matter didn't end with the discovery of the atom. Further research revealed that protons and neutrons are not fundamental particles themselves but are composed of even smaller particles called quarks.
Quarks: The Constituents of Protons and Neutrons
Quarks are fundamental particles that come in six "flavors": up, down, charm, strange, top, and bottom. Protons are composed of two up quarks and one down quark, while neutrons are composed of one up quark and two down quarks. The strong nuclear force, mediated by gluons, holds these quarks together within protons and neutrons.
Leptons: Including the Familiar Electron
Electrons belong to a class of particles called leptons, which are fundamental particles that do not experience the strong nuclear force. Besides the electron, other leptons include the muon and tau particles, along with their associated neutrinos.
The Standard Model of Particle Physics
The Standard Model of particle physics is a theoretical framework that describes the fundamental constituents of matter and their interactions. It incorporates quarks, leptons, and the force-carrying particles (bosons) that mediate the fundamental forces. The Standard Model has been incredibly successful in explaining a vast range of experimental results, but it does have limitations. For instance, it doesn't include gravity and doesn't explain the existence of dark matter and dark energy, which constitute a significant portion of the universe's mass-energy content.
Isotopes and Their Significance
As mentioned earlier, isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutron number can affect the atom's stability and its radioactive properties. Some isotopes are stable, meaning they don't undergo radioactive decay, while others are unstable and decay over time, emitting radiation in the process. Radioactive isotopes have numerous applications in various fields, including medicine (radiotherapy), archaeology (radiocarbon dating), and industrial processes (tracing).
Molecules and Chemical Bonding
Atoms rarely exist in isolation. They tend to interact with each other to form molecules, which are groups of two or more atoms bonded together. Chemical bonding arises from the electromagnetic force acting between the electrons and nuclei of different atoms. There are several types of chemical bonds, including:
- Ionic Bonds: These bonds form when one atom transfers electrons to another, creating ions that are attracted to each other due to their opposite charges. For example, sodium chloride (NaCl) is formed by the transfer of an electron from a sodium atom to a chlorine atom.
- Covalent Bonds: These bonds form when atoms share electrons to achieve a stable electron configuration. For example, the oxygen molecule (O2) is formed by the sharing of electrons between two oxygen atoms.
- Metallic Bonds: These bonds are found in metals, where electrons are delocalized and shared among many atoms. This gives metals their characteristic properties, such as electrical and thermal conductivity and malleability.
The Importance of Understanding the Basic Building Blocks of Matter
Understanding the basic building blocks of matter has profound implications for various fields, including:
- Medicine: Advances in our understanding of atomic structure and chemical bonding have led to the development of new drugs and therapies.
- Materials Science: The development of new materials with specific properties depends on a thorough understanding of atomic interactions.
- Energy Production: Nuclear energy relies on manipulating the strong nuclear force to release energy from atomic nuclei.
- Technology: The miniaturization of electronic devices relies on our understanding of quantum mechanics and the behavior of electrons in materials.
- Cosmology: Our understanding of the formation and evolution of the universe depends on our knowledge of the fundamental particles and forces.
Conclusion: A Journey of Discovery Continues
The journey to understanding the basic building blocks of matter is far from over. While the Standard Model has been incredibly successful, many mysteries remain. The search for new particles and a deeper understanding of the fundamental forces continues to drive scientific research, promising exciting discoveries that will further revolutionize our understanding of the universe and our place within it. From the tiniest quarks to the vast expanse of space, the interconnectedness of all things is a testament to the fundamental building blocks that make up everything we see and experience. The quest for knowledge continues, pushing the boundaries of what we know and opening up new avenues for innovation and discovery.
Latest Posts
Latest Posts
-
Common Factors Of 4 And 10
Mar 06, 2025
-
What Is The Percent Of 2 6
Mar 06, 2025
-
What Is The Lcm Of 3 8
Mar 06, 2025
-
Is A Sound Wave A Transverse Wave
Mar 06, 2025
-
What Is The Electron Configuration For Magnesium
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about The Basic Building Block Of Matter. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
