The Average Kinetic Energy Of The Gas Molecules Is
Juapaving
Mar 23, 2025 · 7 min read
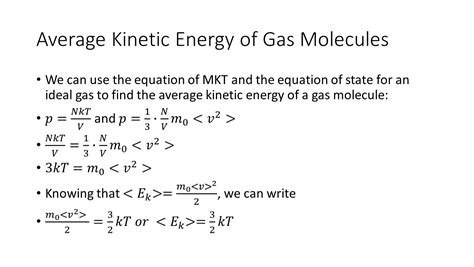
Table of Contents
The Average Kinetic Energy of Gas Molecules: A Deep Dive
The seemingly simple concept of a gas's average kinetic energy hides a wealth of information about the behavior of matter at the microscopic level. Understanding this concept is crucial for comprehending various physical phenomena, from the ideal gas law to the intricacies of thermodynamics. This article delves into the average kinetic energy of gas molecules, exploring its derivation, significance, and applications.
What is Kinetic Energy?
Before diving into the specifics of gas molecules, let's establish a clear understanding of kinetic energy itself. Kinetic energy is the energy an object possesses due to its motion. It's directly proportional to the object's mass (m) and the square of its velocity (v):
KE = ½mv²
This simple equation holds true for macroscopic objects like cars and planets, as well as microscopic particles like gas molecules. However, dealing with a vast number of gas molecules necessitates a statistical approach.
The Kinetic Theory of Gases and its Assumptions
The kinetic theory of gases provides a framework for understanding the macroscopic properties of gases based on the microscopic behavior of their constituent molecules. This theory rests on several key assumptions:
- Large number of particles: Gases contain a vast number of tiny particles (atoms or molecules) in constant, random motion.
- Negligible volume: The volume occupied by the gas molecules themselves is negligible compared to the total volume of the container.
- Elastic collisions: Collisions between gas molecules and the container walls are perfectly elastic, meaning no kinetic energy is lost during collisions.
- No intermolecular forces: There are no significant attractive or repulsive forces between gas molecules. They interact only through collisions.
- Average kinetic energy is proportional to absolute temperature: The average kinetic energy of the gas molecules is directly proportional to the absolute temperature of the gas. This is arguably the most important assumption.
These assumptions, while idealized, provide a surprisingly accurate model for many real gases, especially at low pressures and high temperatures. The degree to which a real gas deviates from ideal behavior is often expressed using the compressibility factor.
Deriving the Average Kinetic Energy
The relationship between the average kinetic energy of gas molecules and temperature is not arbitrary; it can be derived from the postulates of the kinetic theory of gases. A rigorous derivation involves statistical mechanics and Maxwell-Boltzmann distribution, but we can outline the key steps:
-
Pressure and Molecular Collisions: The pressure exerted by a gas is the result of countless molecular collisions with the container walls. Each collision imparts a small change in momentum. The total pressure is proportional to the number of collisions per unit time and the average momentum change per collision.
-
Momentum and Kinetic Energy: The momentum change during a collision is related to the velocity of the molecules. This velocity is directly connected to the kinetic energy of the molecules.
-
Temperature and Average Kinetic Energy: By considering the total pressure, the number of molecules, and their velocities, we can arrive at a relationship that links the average kinetic energy to the absolute temperature (T) of the gas:
KE<sub>avg</sub> = (3/2)kT
Where:
- KE<sub>avg</sub> is the average kinetic energy per molecule
- k is the Boltzmann constant (approximately 1.38 × 10⁻²³ J/K)
- T is the absolute temperature in Kelvin
This equation is a cornerstone of the kinetic theory of gases. It reveals the direct proportionality between average kinetic energy and absolute temperature—as temperature increases, so does the average kinetic energy of the gas molecules.
Significance of Average Kinetic Energy
The concept of average kinetic energy is far from an abstract theoretical concept. It has significant implications across various fields:
-
Ideal Gas Law: The ideal gas law (PV = nRT) can be derived from the kinetic theory of gases, utilizing the relationship between average kinetic energy and temperature. The constant R (the ideal gas constant) incorporates Boltzmann's constant and Avogadro's number, connecting the microscopic world to macroscopic measurements of pressure, volume, and temperature.
-
Diffusion and Effusion: The rates of diffusion (the spreading of a gas throughout a space) and effusion (the escape of a gas through a small hole) are directly related to the average kinetic energy of the gas molecules. Lighter molecules with higher average velocities diffuse and effuse faster than heavier molecules at the same temperature. Graham's Law of Effusion directly reflects this relationship.
-
Temperature Measurement: The concept of temperature itself finds a microscopic interpretation through the average kinetic energy of molecules. A thermometer doesn't directly measure temperature; it measures the effect of the average kinetic energy of molecules within the thermometer on the physical property (expansion) of the liquid within the thermometer.
-
Chemical Reactions: The average kinetic energy of reactant molecules plays a crucial role in determining the rate of chemical reactions. For a reaction to occur, molecules need to collide with sufficient energy (activation energy) to break existing bonds and form new ones. Higher temperatures mean a greater proportion of molecules possessing sufficient activation energy, resulting in a faster reaction rate. This is reflected in the Arrhenius equation.
-
Thermodynamics: The average kinetic energy is a fundamental component in understanding various thermodynamic properties like internal energy and enthalpy. These properties are directly related to the total kinetic energy of the molecules in a system.
-
Atmospheric Science: The kinetic energy of atmospheric gases plays a vital role in weather patterns and climate modeling. Wind is essentially the macroscopic manifestation of the movement of gas molecules; its speed and direction are influenced by variations in the average kinetic energy at different locations within the atmosphere.
Beyond the Ideal Gas: Real Gases and Deviations
The ideal gas law and the directly proportional relationship between average kinetic energy and temperature hold true for ideal gases, gases that conform perfectly to the assumptions of the kinetic theory. Real gases, however, deviate from ideal behavior, particularly at high pressures and low temperatures.
At high pressures, the volume occupied by the gas molecules themselves becomes significant, contradicting one of the core assumptions of the ideal gas model. Additionally, at low temperatures, intermolecular forces become more influential, leading to deviations from ideal behavior. These deviations are accounted for by using equations of state that go beyond the simplistic ideal gas law, such as the van der Waals equation.
Even with these deviations, the concept of average kinetic energy remains a powerful tool for understanding the behavior of real gases. The deviations are generally quantified and incorporated into more refined models, allowing for more accurate predictions of gas properties.
Applications in Various Fields
The concept of average kinetic energy is not confined to theoretical physics. Its practical applications span a wide range of disciplines:
-
Engineering: Understanding the average kinetic energy of gases is crucial in various engineering applications, including designing engines, compressors, and pipelines. Accurate calculations are essential for ensuring efficient and safe operation.
-
Materials Science: The kinetic energy of gas molecules plays a critical role in processes such as chemical vapor deposition (CVD), a technique used to create thin films by reacting gaseous precursors on a substrate surface. Controlling the temperature and therefore the average kinetic energy of the gas molecules is crucial for optimizing film quality and properties.
-
Medicine: The understanding of kinetic energy of gas molecules is important in medical devices and therapies using gases, such as in respiratory therapies, anesthetics, and inhalers. Efficient delivery and distribution require a deep understanding of the behavior of gases.
-
Environmental Science: Atmospheric modeling and pollution studies rely heavily on kinetic theory. Understanding the movement and diffusion of pollutants in the atmosphere is crucial for effective pollution control strategies.
Conclusion: A Fundamental Concept with Broad Reach
The average kinetic energy of gas molecules is a deceptively simple yet profoundly powerful concept. It links the microscopic world of atoms and molecules to the macroscopic properties of gases, providing a fundamental basis for understanding many physical phenomena. From the ideal gas law to the complexities of real-gas behavior, from everyday weather patterns to advanced engineering applications, the concept of average kinetic energy remains an indispensable tool for scientists and engineers alike. The deeper one delves into this concept, the more one appreciates its far-reaching implications and its central role in our understanding of the physical world.
Latest Posts
Latest Posts
-
How Many Hours Is 210 Minutes
Mar 25, 2025
-
Common Factors Of 20 And 40
Mar 25, 2025
-
How Many Lines Of Symmetry Does A Rectangle Has
Mar 25, 2025
-
Lowest Common Denominator Of 9 And 12
Mar 25, 2025
-
Is 15 A Prime Or Composite Number
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about The Average Kinetic Energy Of The Gas Molecules Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
