The Attractive Force Of Water Molecules Is Called The
Juapaving
Mar 06, 2025 · 6 min read
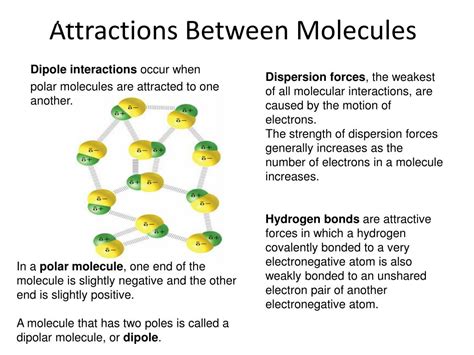
Table of Contents
The Attractive Force of Water Molecules: Understanding Hydrogen Bonding
Water. It's the elixir of life, the solvent of countless reactions, and a substance so ubiquitous we often take it for granted. But the seemingly simple molecule, H₂O, possesses remarkable properties stemming from a powerful intermolecular force: hydrogen bonding. This article delves deep into the nature of this attractive force, exploring its origins, consequences, and significance across various scientific disciplines.
What is Hydrogen Bonding?
Hydrogen bonding is a special type of dipole-dipole attraction between molecules, not a true chemical bond. It occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. This electronegativity difference creates a significant polarity within the water molecule. The oxygen atom, being more electronegative, pulls the shared electrons closer to itself, creating a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogen atoms.
The Role of Electronegativity
The concept of electronegativity is crucial in understanding hydrogen bonding. Electronegativity is an atom's ability to attract electrons within a chemical bond. Oxygen is highly electronegative, meaning it strongly attracts the electrons shared with the hydrogen atoms in a water molecule. This uneven distribution of charge creates a polar molecule, with a positive end (the hydrogen atoms) and a negative end (the oxygen atom).
Hydrogen Bonds vs. Covalent Bonds
It's vital to distinguish between hydrogen bonds and covalent bonds. Covalent bonds involve the sharing of electrons between atoms, forming a strong, intramolecular link. Hydrogen bonds, on the other hand, are intermolecular forces, weaker attractions between separate molecules. They are significantly weaker than covalent bonds but still strong enough to influence many of water's unique properties.
The Strength and Properties of Hydrogen Bonds
While weaker than covalent bonds, hydrogen bonds are considerably stronger than other intermolecular forces like van der Waals forces. This strength arises from the large electronegativity difference between the hydrogen and the electronegative atom, leading to a substantial partial charge separation. The energy required to break a hydrogen bond is typically in the range of 5-30 kJ/mol, significantly less than the energy required to break a covalent bond (hundreds of kJ/mol).
The Impact on Water's Properties
The presence of extensive hydrogen bonding networks significantly impacts water's properties:
-
High Boiling Point: Water has an unusually high boiling point compared to other hydrides in its group (e.g., H₂S, H₂Se). This is because the energy required to overcome the strong hydrogen bonds holding the water molecules together is substantial.
-
High Surface Tension: The cohesive forces due to hydrogen bonding give water a high surface tension, allowing insects to walk on water and contributing to the formation of droplets.
-
High Specific Heat Capacity: Water's high specific heat capacity means it can absorb a large amount of heat without a significant temperature change. This is crucial for regulating temperature in organisms and the environment.
-
High Heat of Vaporization: A large amount of heat energy is needed to convert liquid water to vapor because the hydrogen bonds must be broken. This property is essential for evaporative cooling in organisms.
-
Density Anomaly: Ice is less dense than liquid water, a unique property due to the arrangement of water molecules in the ice crystal lattice. This creates a rigid, open structure with more space between molecules than in liquid water.
-
Excellent Solvent: Water's polarity allows it to dissolve many ionic and polar substances, making it an excellent solvent for biological and chemical processes. The hydrogen bonds help to stabilize dissolved ions and molecules.
Hydrogen Bonding in Biological Systems
Hydrogen bonding plays a pivotal role in numerous biological processes:
-
Protein Structure: Hydrogen bonds are crucial for maintaining the secondary, tertiary, and quaternary structures of proteins. They stabilize alpha-helices, beta-sheets, and the overall three-dimensional folding of proteins, determining their function.
-
DNA Structure: The double helix structure of DNA is stabilized by hydrogen bonds between complementary base pairs (adenine-thymine and guanine-cytosine). These bonds are essential for DNA replication and transcription.
-
Enzyme-Substrate Interactions: Hydrogen bonds contribute to the specific binding of enzymes to their substrates, facilitating enzymatic reactions.
-
Cell Membrane Structure: Hydrogen bonding between water molecules and polar head groups of phospholipids influences the structure and fluidity of cell membranes.
-
Carbohydrate Structure: Hydrogen bonds play a significant role in stabilizing the complex structures of carbohydrates, influencing their properties and functions.
Hydrogen Bonding in Other Systems
Beyond biology, hydrogen bonding is essential in various fields:
-
Material Science: The properties of many materials, including polymers, are significantly affected by hydrogen bonding. Understanding and manipulating hydrogen bonds allows for the design of materials with specific properties.
-
Chemistry: Hydrogen bonding is crucial for understanding the solubility, reactivity, and other properties of many chemical compounds.
-
Atmospheric Science: Hydrogen bonding in water vapor plays a significant role in atmospheric processes, including cloud formation and precipitation.
-
Environmental Science: Hydrogen bonding influences the properties of water in various environmental contexts, such as soil moisture and water pollution.
Further Exploration of Hydrogen Bonding Research
Ongoing research continues to explore the intricacies of hydrogen bonding. Areas of current interest include:
-
Quantum Mechanical Studies: Advanced computational techniques are used to accurately model and predict hydrogen bond strength and geometry.
-
Hydrogen Bonding in Confined Environments: The behavior of hydrogen bonds in nanoscale systems and confined environments is being investigated to understand their impact on material properties.
-
Hydrogen Bond Dynamics: Studies of hydrogen bond dynamics aim to understand the temporal aspects of hydrogen bond formation and breakage, impacting reaction rates and other properties.
-
Applications in Drug Design: Researchers are exploring ways to utilize hydrogen bonds in the design of new drugs and therapeutic agents.
Conclusion: The Significance of Hydrogen Bonding
The attractive force between water molecules, hydrogen bonding, is far more than a simple intermolecular interaction. It's a fundamental force shaping the properties of water and influencing countless processes in biological and non-biological systems. From the intricate structures of proteins and DNA to the macroscopic properties of water, hydrogen bonding plays a vital role, making it a cornerstone concept in diverse scientific disciplines. Its continued study promises further advancements in our understanding of the natural world and offers possibilities for innovative applications in various fields. The seemingly simple water molecule, held together by strong covalent bonds and linked by networks of even more crucial hydrogen bonds, remains a source of endless fascination and scientific discovery. The importance of this seemingly small force cannot be overstated. It truly is the force that shapes our world.
Latest Posts
Latest Posts
-
Where Is The Field Strongest For A Magnet
Mar 07, 2025
-
What Is The Percentage Of 2 8
Mar 07, 2025
-
Alpha Helices And Beta Pleated Sheets Form A Proteins
Mar 07, 2025
-
In The Modern Periodic Table Elements Are Arranged By
Mar 07, 2025
-
The Product Of Two Irrational Numbers Is
Mar 07, 2025
Related Post
Thank you for visiting our website which covers about The Attractive Force Of Water Molecules Is Called The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
