Alpha Helices And Beta Pleated Sheets Form A Protein's
Juapaving
Mar 07, 2025 · 7 min read
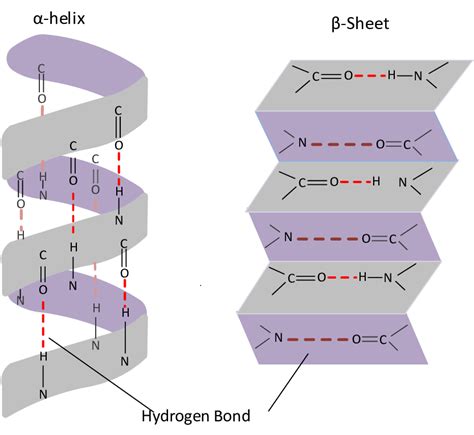
Table of Contents
Alpha Helices and Beta Pleated Sheets: The Building Blocks of a Protein's Structure
Proteins are the workhorses of the cell, carrying out a vast array of functions essential for life. Their incredible versatility stems from their diverse three-dimensional structures, which are ultimately determined by their amino acid sequences. While the sequence dictates the potential structure, the actual folding into a functional protein involves a complex interplay of forces, leading to intricate arrangements of secondary structures like alpha helices and beta pleated sheets. Understanding these secondary structures is crucial to grasping the overall architecture and function of proteins.
What are Secondary Structures?
Before diving into the specifics of alpha helices and beta sheets, it's important to define secondary structure. This level of protein organization refers to local folding patterns of the polypeptide chain, stabilized by hydrogen bonds between the backbone amide and carbonyl groups. These local folds are relatively regular and repetitive, unlike the more complex and irregular tertiary structure which encompasses the overall 3D arrangement of the entire polypeptide chain. Alpha helices and beta pleated sheets are the two most common types of secondary structures, though others, like loops and turns, also exist and contribute to the overall protein architecture.
Alpha Helices: A Spiraling Structure
The alpha helix is a right-handed coiled conformation, resembling a spring or a spiral staircase. This structure is stabilized by hydrogen bonds formed between the carbonyl oxygen of one amino acid and the amide hydrogen of the amino acid four residues further along the chain. This specific spacing (i-4 interaction) creates a regular repeating pattern, resulting in a tightly packed helix with 3.6 amino acid residues per turn.
Key Features of Alpha Helices:
-
Hydrogen Bonding: The backbone hydrogen bonds are crucial for stability, creating a strong, cohesive structure. The presence of proline residues, which lack the amide hydrogen required for hydrogen bonding, often disrupts alpha helix formation. Similarly, the presence of charged amino acids can also impact stability depending on their interactions.
-
Side Chain Orientation: The side chains of the amino acids extend outward from the helix, minimizing steric hindrance. This orientation contributes to the overall stability and interactions with the protein's environment.
-
Dipole Moment: Due to the arrangement of peptide bonds, alpha helices possess a net dipole moment, with the N-terminus being more positive and the C-terminus being more negative. This inherent polarity can influence interactions with other molecules or regions within the protein.
-
Amphipathic Helices: Some alpha helices are amphipathic, meaning they possess both hydrophobic and hydrophilic regions. This characteristic often plays a significant role in membrane protein structure, where the hydrophobic region interacts with the lipid bilayer and the hydrophilic region interacts with the aqueous environment.
Beta Pleated Sheets: A Flattened Structure
Unlike the coiled alpha helix, beta pleated sheets are characterized by a flattened, zig-zag arrangement of polypeptide chains. These chains, often referred to as beta strands, are linked together laterally by hydrogen bonds between adjacent strands. These hydrogen bonds form between the carbonyl oxygen of one strand and the amide hydrogen of the adjacent strand.
Key Features of Beta Pleated Sheets:
-
Hydrogen Bonding: The hydrogen bonds between adjacent strands are the primary stabilizing force in beta sheets. The strength and extent of these bonds depend on the length and regularity of the strands.
-
Parallel and Antiparallel Sheets: Beta sheets can be either parallel or antiparallel, depending on the orientation of the participating strands. In parallel beta sheets, the N-termini of all strands are aligned in the same direction. In antiparallel beta sheets, the N-terminus of one strand is aligned with the C-terminus of the adjacent strand. Antiparallel sheets are generally more stable due to the more linear hydrogen bonds they form.
-
Side Chain Orientation: Side chains in beta sheets alternate above and below the plane of the sheet. This arrangement is important for interactions with other parts of the protein or with other molecules.
-
Beta Turns and Loops: Beta sheets are often connected by loops and turns, which provide flexibility and allow the sheet to fold into a specific three-dimensional structure. These turns and loops are typically short and have specific amino acid compositions that contribute to their structure and stability.
The interplay between Alpha Helices and Beta Pleated Sheets in Protein Structure
Alpha helices and beta pleated sheets are not mutually exclusive; many proteins incorporate both secondary structures in their overall architecture. The arrangement and proportions of these secondary structures significantly contribute to the protein's tertiary structure and overall function. The spatial organization of these elements is driven by several factors:
-
Hydrophobic Interactions: Hydrophobic amino acid side chains tend to cluster together in the protein's interior, away from the aqueous environment. This clustering often leads to the formation of alpha helices or beta sheets with hydrophobic surfaces facing inward.
-
Hydrophilic Interactions: Conversely, hydrophilic amino acid side chains are often found on the protein's surface, interacting with water molecules.
-
Disulfide Bonds: Covalent disulfide bonds between cysteine residues can further stabilize the overall protein structure, including the arrangement of alpha helices and beta sheets. These bonds are crucial in many proteins for maintaining their functional conformation, particularly those exposed to harsh extracellular conditions.
-
Electrostatic Interactions: Interactions between charged amino acid residues can also contribute to the overall protein fold. Attractive interactions between oppositely charged residues can stabilize the structure while repulsive interactions between similarly charged residues can influence the folding pathways.
Examples of Proteins with Different Proportions of Alpha Helices and Beta Sheets
The relative proportions of alpha helices and beta sheets vary significantly among different proteins. This variation reflects the diverse functions proteins perform.
-
Globular Proteins: Many globular proteins, like enzymes, contain a mixture of alpha helices and beta sheets, often arranged in a compact, spherical shape. Myoglobin, a protein responsible for oxygen storage in muscle tissue, is a classic example. Its structure consists primarily of alpha-helices.
-
Fibrous Proteins: Fibrous proteins, like collagen and keratin, often consist predominantly of either alpha helices or beta sheets, arranged in long, extended structures. Collagen, a major component of connective tissue, features a triple helix structure, while keratin, found in hair and nails, is rich in alpha-helices.
-
Membrane Proteins: Membrane proteins often have transmembrane alpha helices that span the lipid bilayer, with hydrophilic regions exposed to the aqueous environment and hydrophobic regions embedded within the membrane. The arrangement of these helices is critical for their function in transporting molecules across the cell membrane.
Predicting Secondary Structure
Predicting the secondary structure of a protein from its amino acid sequence is a challenging but crucial area of bioinformatics. Various algorithms and prediction methods have been developed, based on statistical analysis of known protein structures and the physicochemical properties of amino acids. These methods vary in accuracy, but they provide valuable insights into the potential secondary structure elements within a protein. While computational prediction is a powerful tool, experimental methods remain crucial for verifying predicted structures and revealing the intricate details of protein folding.
Studying Alpha Helices and Beta Pleated Sheets: Experimental Techniques
Various experimental techniques are employed to study the structure and dynamics of alpha helices and beta pleated sheets.
-
X-ray crystallography: This technique provides high-resolution images of protein structures, revealing the precise arrangement of atoms within alpha helices and beta sheets. It is a powerful tool for determining the detailed 3D structure but requires obtaining high-quality protein crystals.
-
Nuclear Magnetic Resonance (NMR) spectroscopy: NMR spectroscopy provides information about protein structure and dynamics in solution. It's particularly useful for studying flexible regions and dynamic interactions within the protein, offering a complementary approach to crystallography.
-
Circular Dichroism (CD) spectroscopy: CD spectroscopy is a valuable technique for analyzing the secondary structure content of proteins. It can be used to estimate the proportions of alpha helices and beta sheets in a protein sample without the need for crystallization or high concentrations.
Conclusion: The Significance of Secondary Structure
Alpha helices and beta pleated sheets are fundamental structural motifs in proteins, contributing significantly to their diverse functions. Understanding their properties, formation, and interactions is essential for comprehending how proteins fold, interact, and carry out their biological roles. The ongoing research in protein structure continues to unveil new insights into the complex interplay between amino acid sequence, secondary structure, and the remarkable functional diversity of proteins. Advancements in computational and experimental techniques are crucial in uncovering the secrets of protein folding and pushing the boundaries of our understanding of life's molecular machinery. The study of these secondary structures is fundamental to the fields of biochemistry, molecular biology, and medicine, enabling drug design, protein engineering, and the advancement of our comprehension of biological processes at the molecular level.
Latest Posts
Latest Posts
-
What Is The Function Of Base In Microscope
Mar 11, 2025
-
The Rows In The Periodic Table Are Called
Mar 11, 2025
-
Salicylic Acid React With Acetic Anhydride
Mar 11, 2025
-
A Database Is A Collection Of Related Data
Mar 11, 2025
-
Is Tap Water A Mixture Or Pure Substance
Mar 11, 2025
Related Post
Thank you for visiting our website which covers about Alpha Helices And Beta Pleated Sheets Form A Protein's . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
