The Ability To Do Work Is Called
Juapaving
Mar 20, 2025 · 5 min read
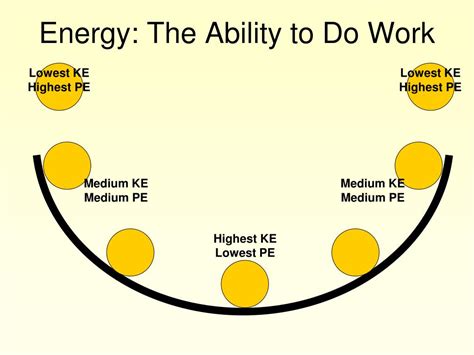
Table of Contents
The Ability to Do Work: Exploring Energy and its Manifestations
The ability to do work is fundamentally defined as energy. This seemingly simple statement underpins our understanding of the universe, from the smallest subatomic particles to the largest galaxies. Understanding energy, its various forms, and its relationship to work is crucial across numerous scientific disciplines, and even permeates our everyday lives. This article will delve deep into the concept of energy and its capacity to perform work, exploring different types of energy, energy transformations, and the crucial role it plays in our world.
What is Work?
Before we dive into the definition of energy, it's essential to understand what constitutes "work" in the context of physics. Work, in physics, is not simply any activity that expends effort. Instead, it's a specific interaction between a force and the displacement of an object. Work is done when a force causes an object to move in the direction of the force.
Mathematically, work (W) is calculated as:
W = Fd cos θ
Where:
- F represents the magnitude of the force applied.
- d represents the displacement of the object.
- θ represents the angle between the force and the displacement.
This equation highlights that work is only done if there's a force applied and a resulting displacement in the direction of the force. For example, holding a heavy box stationary requires effort, but no work is done because there's no displacement. However, lifting the box involves work because a force is applied, causing a displacement.
Energy: The Capacity to Do Work
Now, let's return to the central theme: energy. Energy is the capacity to do work. It's a fundamental property of all matter and represents the potential to cause change or perform an action. Energy is neither created nor destroyed; it simply transforms from one form to another, a principle known as the law of conservation of energy.
This principle is paramount in understanding how energy facilitates work. When energy is transferred or transformed, it can perform work. For instance, the chemical energy stored in gasoline is converted into mechanical energy in a car engine, which then performs the work of moving the vehicle.
Types of Energy
Energy manifests itself in numerous forms, each with unique characteristics and applications:
1. Kinetic Energy: Energy of Motion
Kinetic energy is the energy possessed by an object due to its motion. The faster an object moves, and the greater its mass, the more kinetic energy it possesses. The formula for kinetic energy (KE) is:
KE = ½mv²
Where:
- m represents the mass of the object.
- v represents the velocity of the object.
Examples of kinetic energy include a moving car, a flying airplane, and even the thermal motion of molecules in a hot object.
2. Potential Energy: Stored Energy
Potential energy is stored energy that has the potential to be converted into other forms of energy. There are several types of potential energy:
-
Gravitational Potential Energy: This is the energy stored in an object due to its position relative to a gravitational field. The higher an object is raised above the ground, the greater its gravitational potential energy. The formula is:
GPE = mgh
Where:
- m represents the mass of the object.
- g represents the acceleration due to gravity.
- h represents the height of the object above a reference point.
-
Elastic Potential Energy: This is the energy stored in a stretched or compressed elastic material, such as a spring.
-
Chemical Potential Energy: This is the energy stored in the bonds between atoms and molecules. This energy is released during chemical reactions, such as combustion.
3. Thermal Energy (Heat): Molecular Motion
Thermal energy, or heat, is the total kinetic energy of all the particles in a substance. The higher the temperature of a substance, the greater its thermal energy. Heat transfer occurs when thermal energy flows from a hotter object to a colder object.
4. Radiant Energy (Electromagnetic Radiation): Energy of Light
Radiant energy is energy that travels in the form of electromagnetic waves, including visible light, ultraviolet radiation, infrared radiation, and X-rays. The sun is a primary source of radiant energy, which drives many processes on Earth.
5. Electrical Energy: Flow of Charge
Electrical energy is the energy associated with the flow of electric charge. This energy is used to power numerous devices, from lighting to computers.
6. Nuclear Energy: Energy from Atomic Nuclei
Nuclear energy is the energy stored within the nuclei of atoms. This energy is released during nuclear fission (splitting of atoms) or nuclear fusion (combining of atoms).
7. Sound Energy: Vibrational Energy
Sound energy is the energy carried by sound waves. These waves are produced by vibrating objects and cause vibrations in the surrounding medium, allowing us to hear.
Energy Transformations and Work
The various forms of energy are interconnected and can be transformed from one form to another. This transformation is often accompanied by the performance of work. Consider these examples:
-
Hydroelectric Power: The gravitational potential energy of water stored behind a dam is converted into kinetic energy as the water flows downhill. This kinetic energy then drives turbines, generating electrical energy.
-
Photosynthesis: Plants convert radiant energy from sunlight into chemical potential energy in the form of glucose during photosynthesis.
-
Combustion: The chemical potential energy stored in fuels like wood or gasoline is released as heat and light during combustion. This heat energy can then be used to perform work, as in a car engine.
Energy and Efficiency
The efficiency of an energy transformation process refers to the ratio of useful energy output to the total energy input. No process is 100% efficient; some energy is always lost as heat or other unusable forms. Understanding energy efficiency is crucial for minimizing energy waste and promoting sustainable practices.
Conclusion: Energy and Our World
The ability to do work is inextricably linked to energy. Understanding the various forms of energy, their transformations, and the principle of conservation of energy is fundamental to comprehending the physical world around us. From the smallest biological processes to the largest industrial operations, energy plays an indispensable role. As we continue to develop and refine our understanding of energy, we can strive towards more efficient and sustainable ways to harness its power for the benefit of humankind. The study of energy is not merely an academic pursuit; it is an essential element of technological advancement, economic growth, and the long-term sustainability of our planet. Continued research and innovation in energy production and utilization will undoubtedly shape the future of our world.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 25 And 30
Mar 20, 2025
-
What Is The Least Common Multiple Of 8 And 4
Mar 20, 2025
-
Difference Between Dihybrid And Monohybrid Cross
Mar 20, 2025
-
What Is The Prime Factorization Of 13
Mar 20, 2025
-
Is Ice Cream Melting A Chemical Change
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about The Ability To Do Work Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
