Tap Water Pure Substance Or Mixture
Juapaving
Mar 28, 2025 · 5 min read
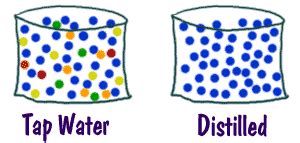
Table of Contents
Is Tap Water a Pure Substance or a Mixture? A Deep Dive into Water Chemistry
Water is essential for life, and we interact with it daily, often without a second thought. But have you ever stopped to consider the actual composition of the water flowing from your tap? Is tap water a pure substance or a mixture? The answer, as we'll explore in detail, is unequivocally a mixture. While it might seem simple, understanding the complex composition of tap water opens a fascinating window into chemistry and the processes involved in making our water safe for consumption. This article delves into the scientific intricacies, examining the various components, the purification processes, and the implications for our health and the environment.
Understanding Pure Substances and Mixtures
Before we dive into the specifics of tap water, let's establish a clear understanding of the fundamental difference between pure substances and mixtures.
Pure substances are composed of only one type of atom or molecule. Examples include elements like oxygen (O₂) and gold (Au), and compounds like water (H₂O) and table salt (NaCl). These substances have a fixed chemical composition and consistent properties.
Mixtures, on the other hand, are composed of two or more substances that are physically combined but not chemically bonded. They can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water). The relative amounts of each component can vary. Crucially, the properties of a mixture are a reflection of the properties of its constituent components.
With this distinction established, it becomes clear why tap water is categorized as a mixture.
The Complex Composition of Tap Water: More Than Just H₂O
While the chemical formula for water is H₂O, implying two hydrogen atoms bonded to one oxygen atom, tap water contains significantly more than just these three elements. The exact composition varies greatly depending on several factors including:
- Source: Water sourced from rivers, lakes, underground aquifers, or reservoirs will have different initial compositions.
- Treatment processes: Different water treatment plants employ varying methods to purify water, influencing the final product.
- Location: Geographic variations in soil and geological formations affect the mineral content of the water.
- Season: Seasonal changes can affect the concentration of certain dissolved substances.
Dissolved Minerals and Ions
Tap water typically contains various dissolved minerals and ions, many of which are essential for human health in moderate amounts. These include:
- Calcium (Ca²⁺): Essential for strong bones and teeth.
- Magnesium (Mg²⁺): Important for muscle function and nerve transmission.
- Sodium (Na⁺): Plays a role in fluid balance and nerve impulses.
- Potassium (K⁺): Essential for maintaining fluid balance and muscle contractions.
- Bicarbonate (HCO₃⁻): Acts as a buffer, helping to maintain the water's pH.
- Sulfate (SO₄²⁻): Can contribute to the taste and hardness of the water.
- Chloride (Cl⁻): Contributes to the salinity of the water.
These minerals originate from the interaction of water with rocks and soil as it travels through the water cycle. The levels of these minerals determine the water's "hardness," with hard water containing higher concentrations of calcium and magnesium.
Gases
Water also dissolves various gases from the atmosphere, including:
- Oxygen (O₂): Essential for aquatic life.
- Carbon dioxide (CO₂): Contributes to the water's pH and can form carbonic acid.
- Nitrogen (N₂): Generally inert in water.
The concentration of these gases can fluctuate depending on factors such as temperature, pressure, and exposure to the atmosphere.
Other Contaminants
Depending on the source and treatment processes, tap water might contain other substances, some of which are considered contaminants:
- Heavy metals: Trace amounts of lead, mercury, or arsenic can be present, although stringent regulations aim to minimize these levels.
- Pesticides and herbicides: Runoff from agricultural lands can contaminate water sources.
- Pharmaceuticals and personal care products: These emerging contaminants are increasingly detected in water supplies.
- Microbial contaminants: Bacteria, viruses, and protozoa can contaminate water sources if not properly treated.
- Dissolved organic matter: This includes humic acids and other organic compounds that can affect the water’s color, taste, and odor.
Water treatment plants employ various methods to remove or reduce these contaminants, ensuring the water is safe for human consumption.
Water Treatment Processes: Transforming a Mixture
The process of transforming raw water into potable tap water involves a series of intricate steps designed to remove or reduce impurities and render the water safe. These processes include:
- Coagulation and Flocculation: Chemicals are added to clump together suspended particles, forming larger flocs that are easier to remove.
- Sedimentation: The heavier flocs settle out of the water in large sedimentation tanks.
- Filtration: Water is passed through layers of sand, gravel, and other filter media to remove remaining suspended particles.
- Disinfection: Chemicals like chlorine, chloramine, or ultraviolet light are used to kill harmful microorganisms.
- pH Adjustment: Acids or bases are added to adjust the water's pH to an optimal range.
These treatments effectively modify the composition of the water, removing or reducing the concentration of many contaminants while leaving behind a mixture that is considered safe for human consumption. However, it remains a mixture, not a pure substance.
The Implications for Health and the Environment
The composition of tap water directly impacts both human health and the environment.
Health: The presence of essential minerals like calcium and magnesium in moderate quantities is beneficial, while excessive amounts of certain minerals or the presence of harmful contaminants can pose health risks. Regular monitoring of tap water quality is crucial to ensure it meets safety standards.
Environment: Water treatment processes can generate byproducts, and the discharge of treated wastewater can have environmental consequences. Minimizing the environmental impact of water treatment is a major focus for water utilities.
Conclusion: A Mixture We Rely On
In conclusion, tap water is undeniably a mixture, not a pure substance. It's a complex solution containing dissolved minerals, gases, and potentially other contaminants, all influenced by its source, treatment processes, and geographic location. While the water treatment process aims to remove or reduce harmful substances, the resulting water still contains many different components. Understanding this complex composition is crucial for appreciating the intricate processes involved in providing safe, potable water and for promoting responsible water management practices. The composition of our tap water is a testament to the science and technology behind our daily necessities, highlighting the need for ongoing research and vigilance to ensure safe and sustainable water resources for generations to come. This continuous monitoring and improvement are key to ensuring the quality and safety of the mixture we so readily consume.
Latest Posts
Latest Posts
-
Lcm Of 9 And 12 And 15
Mar 31, 2025
-
Which Of The Following Processes Returns Carbon To The Atmosphere
Mar 31, 2025
-
5 Letter Word That Starts With Vi
Mar 31, 2025
-
Can Acquired Characteristics Be Passed On The Next Generation
Mar 31, 2025
-
What Are The Three Body Parts Of A Mollusk
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Tap Water Pure Substance Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
