Strength Of A Solution In Chemistry
Juapaving
Mar 16, 2025 · 6 min read
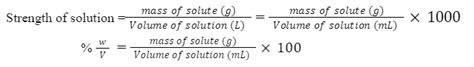
Table of Contents
Strength of a Solution in Chemistry: A Comprehensive Guide
Understanding the strength of a solution is fundamental in chemistry, impacting various fields from medicine and environmental science to industrial processes. This comprehensive guide delves deep into the concept, exploring different types of solution strength, their calculations, and their significance in different applications.
What is Solution Strength?
In chemistry, the strength of a solution refers to the concentration of a solute dissolved in a solvent. It quantifies the amount of solute present in a given amount of solution. A stronger solution has a higher concentration of solute, while a weaker solution has a lower concentration. This seemingly simple concept manifests in several ways, leading to different ways of expressing solution strength.
Key Terms: Solute, Solvent, and Solution
Before we delve into the specifics, let's clarify some crucial terminology:
-
Solute: This is the substance that is being dissolved. It's usually present in a smaller amount compared to the solvent. Examples include salt (NaCl), sugar (C₁₂H₂₂O₁₁), and various gases.
-
Solvent: This is the substance that dissolves the solute. It's usually present in a larger amount. Water is the most common solvent, but others include ethanol, acetone, and benzene.
-
Solution: This is the homogeneous mixture formed when the solute dissolves in the solvent. It's crucial to remember that the solution is uniformly mixed at the molecular level.
Expressing Solution Strength: Different Methods
Several methods exist for expressing the strength or concentration of a solution. Each method has its advantages and disadvantages, making it suitable for specific applications. The choice depends on the context and the information needed.
1. Molarity (M)
Molarity is arguably the most common way to express solution concentration. It's defined as the number of moles of solute per liter of solution.
Formula: Molarity (M) = moles of solute / liters of solution
Example: A solution containing 0.5 moles of NaCl in 1 liter of water has a molarity of 0.5 M (0.5 molar).
Advantages: Molarity is straightforward to calculate and widely used in chemical calculations, especially those involving stoichiometry.
Disadvantages: Molarity is temperature-dependent. As temperature changes, the volume of the solution can change, thereby affecting the molarity.
2. Molality (m)
Molality is defined as the number of moles of solute per kilogram of solvent. Unlike molarity, it's independent of temperature changes.
Formula: Molality (m) = moles of solute / kilograms of solvent
Example: A solution containing 0.5 moles of NaCl in 1 kg of water has a molality of 0.5 m (0.5 molal).
Advantages: Molality is temperature-independent, making it more precise for certain applications, particularly those involving temperature variations.
Disadvantages: Molality can be slightly more complex to calculate than molarity, requiring precise measurement of solvent mass.
3. Normality (N)
Normality is defined as the number of gram-equivalent weights of solute per liter of solution. This method is particularly useful in acid-base titrations and redox reactions. The gram-equivalent weight depends on the reaction the solute is involved in. For acids, it's the molecular weight divided by the number of acidic protons; for bases, it's the molecular weight divided by the number of hydroxide ions.
Formula: Normality (N) = gram-equivalent weight of solute / liters of solution
Advantages: Normality simplifies calculations in titrations where equivalent weights are crucial.
Disadvantages: Normality is highly reaction-dependent. The same solution can have different normalities depending on the specific chemical reaction it participates in. This makes it less versatile than molarity or molality.
4. Percent Concentration (% w/v, % w/w, % v/v)
Percent concentration expresses the amount of solute as a percentage of the total solution or solvent. There are three main types:
- Percent weight/volume (% w/v): grams of solute per 100 mL of solution.
- Percent weight/weight (% w/w): grams of solute per 100 grams of solution.
- Percent volume/volume (% v/v): milliliters of solute per 100 mL of solution. This is commonly used for liquid solutes dissolved in liquid solvents.
Advantages: Percent concentration is easy to understand and use, making it suitable for everyday applications.
Disadvantages: It lacks the precision of molarity and molality for chemical calculations, and the type of percentage must be clearly specified.
5. Parts Per Million (ppm) and Parts Per Billion (ppb)
These units express very low concentrations of solutes, typically used for trace amounts of substances in environmental science and analytical chemistry.
- Parts per million (ppm): milligrams of solute per liter of solution (or milligrams of solute per kilogram of solution).
- Parts per billion (ppb): micrograms of solute per liter of solution (or micrograms of solute per kilogram of solution).
Advantages: ppm and ppb are practical for representing extremely low concentrations.
Disadvantages: They might be less intuitive for those unfamiliar with the units, and their value can differ slightly based on whether it's mg/L or mg/kg etc.
Calculations and Examples
Let's look at some examples to illustrate the calculations of different solution strengths.
Example 1: Calculating Molarity
Suppose we dissolve 58.5 grams of NaCl (molecular weight = 58.5 g/mol) in enough water to make 1 liter of solution. What is the molarity of the solution?
First, we calculate the number of moles of NaCl:
Moles of NaCl = mass / molecular weight = 58.5 g / 58.5 g/mol = 1 mol
Then, we calculate the molarity:
Molarity = moles of solute / liters of solution = 1 mol / 1 L = 1 M
Example 2: Calculating Molality
If we dissolve 58.5 grams of NaCl in 1 kg of water, what's the molality?
Moles of NaCl = 1 mol (as calculated above)
Molality = moles of solute / kilograms of solvent = 1 mol / 1 kg = 1 m
Example 3: Calculating Percent Concentration (% w/v)
If we dissolve 10 grams of sugar in 100 mL of water, what's the % w/v concentration?
% w/v = (grams of solute / mL of solution) x 100 = (10 g / 100 mL) x 100 = 10% w/v
Significance of Solution Strength in Different Applications
Understanding solution strength is crucial across many scientific and practical disciplines:
1. Medicine and Pharmacy
Accurate solution strengths are vital in preparing medications. Incorrect concentrations can lead to ineffective treatment or even toxicity. Intravenous fluids, for instance, require precise molarity to maintain the patient's electrolyte balance.
2. Environmental Science
Measuring the strength of pollutants in water bodies, soil, and air is critical for environmental monitoring and remediation. ppm and ppb are commonly used to express concentrations of contaminants like heavy metals and pesticides.
3. Industrial Processes
Many industrial processes involve solutions of specific concentrations. For example, the strength of acids and bases used in manufacturing processes must be carefully controlled to ensure product quality and safety.
4. Analytical Chemistry
Accurate determination of solution strength is fundamental in various analytical techniques like titration, spectrophotometry, and chromatography. These techniques rely on precise concentrations for accurate and reliable results.
5. Food and Beverage Industry
Solution strength is crucial in food and beverage production. The concentration of sugar, salt, and acids affects taste, preservation, and texture.
Conclusion
The strength of a solution is a fundamental concept in chemistry with far-reaching implications. Understanding the different ways to express solution concentration, their calculations, and their importance in various applications is essential for anyone working in chemistry-related fields. The choice of method for expressing concentration depends heavily on the specific application and the level of precision required. From the simple percent concentration used in everyday life to the precise molarity used in complex chemical reactions, a firm grasp of this concept is key to accurate experimentation, safe practices, and effective problem-solving in a variety of scientific disciplines.
Latest Posts
Latest Posts
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
-
Does Cold Air Go Up Or Down
Mar 17, 2025
-
Least Common Multiple Of 20 And 3
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Strength Of A Solution In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
