Sodium Carbonate + Hydrochloric Acid Balanced Equation
Juapaving
Mar 23, 2025 · 6 min read
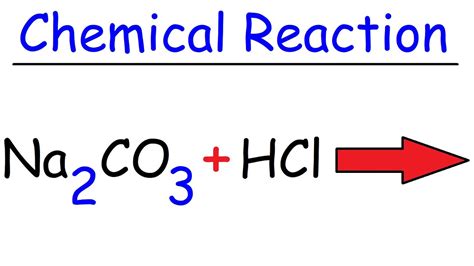
Table of Contents
Sodium Carbonate + Hydrochloric Acid: A Deep Dive into the Balanced Equation and Beyond
The reaction between sodium carbonate (Na₂CO₃) and hydrochloric acid (HCl) is a classic example of an acid-base reaction, frequently encountered in chemistry classrooms and various industrial applications. Understanding this reaction, from its balanced equation to its implications, is crucial for anyone studying chemistry or working in related fields. This comprehensive article delves into the intricacies of this reaction, exploring its balanced equation, the underlying chemistry, practical applications, and safety considerations.
Understanding the Reactants: Sodium Carbonate and Hydrochloric Acid
Before diving into the reaction itself, let's briefly examine the individual reactants:
Sodium Carbonate (Na₂CO₃):
Sodium carbonate, also known as washing soda or soda ash, is a white, crystalline powder that is highly soluble in water. It's a common ingredient in many household cleaning products due to its alkaline nature and ability to soften water. Its chemical structure involves a carbonate anion (CO₃²⁻) bound to two sodium cations (Na⁺). This carbonate ion is responsible for its characteristic basicity.
Hydrochloric Acid (HCl):
Hydrochloric acid is a strong, corrosive acid, a solution of hydrogen chloride (HCl) in water. It's a vital reagent in many industrial processes and laboratory settings. HCl is a strong acid because it readily dissociates in water to produce hydrogen ions (H⁺) and chloride ions (Cl⁻). These hydrogen ions are the key to its acidic properties.
The Balanced Chemical Equation
The reaction between sodium carbonate and hydrochloric acid is a neutralization reaction, where an acid and a base react to form a salt and water. The reaction proceeds in two distinct steps:
Step 1:
Na₂CO₃(aq) + HCl(aq) → NaHCO₃(aq) + NaCl(aq)
In this first step, one mole of hydrochloric acid reacts with one mole of sodium carbonate to produce one mole of sodium bicarbonate (NaHCO₃), also known as baking soda, and one mole of sodium chloride (NaCl), common table salt. This is a relatively slow reaction.
Step 2:
NaHCO₃(aq) + HCl(aq) → NaCl(aq) + H₂O(l) + CO₂(g)
The sodium bicarbonate produced in the first step then reacts with another mole of hydrochloric acid to produce more sodium chloride, water (H₂O), and carbon dioxide gas (CO₂). This step is faster and characterized by the evolution of carbon dioxide gas, which is observable as effervescence.
Overall Balanced Equation:
Combining both steps, the overall balanced chemical equation for the reaction of sodium carbonate with hydrochloric acid is:
Na₂CO₃(aq) + 2HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g)
This equation shows that one mole of sodium carbonate reacts with two moles of hydrochloric acid to produce two moles of sodium chloride, one mole of water, and one mole of carbon dioxide gas. The stoichiometry is crucial for understanding the quantitative aspects of the reaction.
Understanding the Reaction Mechanism
The reaction occurs through a series of proton transfer steps. The carbonate ion (CO₃²⁻) acts as a Brønsted-Lowry base, accepting protons (H⁺) from the hydrochloric acid. The first protonation forms the bicarbonate ion (HCO₃⁻), which is then further protonated to form carbonic acid (H₂CO₃). However, carbonic acid is unstable and readily decomposes into water and carbon dioxide, leading to the observable effervescence.
This mechanism explains why the reaction proceeds in two steps and why carbon dioxide is produced. The overall reaction represents the complete neutralization of the carbonate ion.
Applications of the Reaction
The reaction between sodium carbonate and hydrochloric acid has several important applications across various fields:
1. Quantitative Analysis:
This reaction finds significant application in quantitative analysis, particularly in acid-base titrations. By accurately measuring the volume of hydrochloric acid required to neutralize a known amount of sodium carbonate, the concentration of the acid can be determined. This principle is used extensively in chemistry laboratories for determining the concentration of unknown acid solutions.
2. Industrial Processes:
In the industrial setting, this reaction plays a role in various processes. For instance, it can be used to adjust the pH of solutions, remove carbonate impurities, or generate carbon dioxide. The reaction’s ability to produce carbon dioxide has applications in the production of carbonated beverages and other processes requiring controlled CO₂ release.
3. Chemical Synthesis:
The reaction can also be a crucial step in certain chemical syntheses where the production of sodium chloride or the controlled release of carbon dioxide are desired. This controlled release is advantageous in specific chemical processes requiring precise reaction conditions.
4. Cleaning and Decalcification:
Due to its ability to neutralize acids and dissolve carbonates, this reaction has applications in cleaning and decalcification processes. This is particularly relevant in industrial settings dealing with hard water or mineral deposits.
Safety Precautions
When handling hydrochloric acid and sodium carbonate, several safety precautions must be observed:
- Hydrochloric acid is highly corrosive: Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat. Handle with care and avoid contact with skin and eyes.
- Carbon dioxide is produced: The reaction produces carbon dioxide gas. Ensure adequate ventilation to prevent the buildup of CO₂, which can displace oxygen and cause respiratory problems.
- Exothermic Reaction: The reaction is exothermic, meaning it releases heat. Perform the reaction in a suitable container and take appropriate measures to control the temperature if large quantities are involved.
- Waste Disposal: Dispose of the reaction waste properly according to local regulations. Hydrochloric acid and its reaction products should not be released into the environment without proper treatment.
Further Considerations: Variations and Extensions
While the above details cover the core aspects of the reaction, several factors can influence its outcome:
- Concentration of reactants: The rate of the reaction is dependent on the concentration of both sodium carbonate and hydrochloric acid. Higher concentrations lead to a faster reaction rate.
- Temperature: Increasing the temperature generally accelerates the reaction rate.
- Presence of other ions: The presence of other ions in the solution may affect the reaction rate and equilibrium.
Understanding these factors is important for controlling and optimizing the reaction in practical applications.
Conclusion
The reaction between sodium carbonate and hydrochloric acid is a fundamental chemical process with broad implications across various scientific and industrial fields. From its balanced equation to its practical applications and safety considerations, understanding this reaction is paramount for chemists, chemical engineers, and anyone working in related areas. By mastering the principles of this reaction, one gains a deeper understanding of acid-base chemistry and its importance in various practical contexts. The ability to predict and control the outcome of this reaction is essential for efficient and safe chemical processes. This detailed explanation aims to provide a comprehensive understanding of the topic, enabling readers to apply their knowledge in diverse settings.
Latest Posts
Latest Posts
-
How Many Sides Does A Rectangle Have
Mar 25, 2025
-
Litmus Paper Test For Acid And Base
Mar 25, 2025
-
What Is The Percent Of 1 25
Mar 25, 2025
-
What Is The Magnitude Of A Force
Mar 25, 2025
-
How Many Feet Is 37 Inches
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Sodium Carbonate + Hydrochloric Acid Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
