Single Displacement Reaction Examples In Everyday Life
Juapaving
Mar 19, 2025 · 6 min read
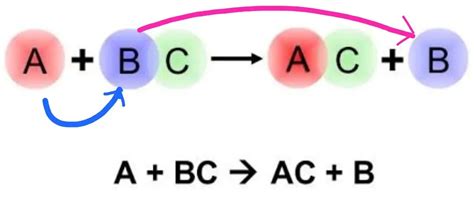
Table of Contents
Single Displacement Reactions: Everyday Examples You Might Not Notice
Single displacement reactions, also known as single replacement reactions, are a fundamental type of chemical reaction where one element replaces another element in a compound. These reactions are surprisingly common in everyday life, often unnoticed but impacting various aspects of our daily routines. This article will explore numerous examples of single displacement reactions, explaining the underlying chemistry and highlighting their relevance in various contexts. We will delve into the core principles, explore diverse examples, and even touch on the broader implications of these reactions.
Understanding the Basics of Single Displacement Reactions
Before diving into the practical examples, let's briefly revisit the underlying principles. A single displacement reaction follows a general pattern:
A + BC → AC + B
Here, A is a more reactive element than B. The reactivity is determined by the activity series of metals (or the electronegativity series for nonmetals). A more reactive element will readily displace a less reactive element from its compound. This reaction will only proceed if A is higher on the activity series than B.
Everyday Examples of Single Displacement Reactions
Now, let's explore the diverse ways single displacement reactions manifest in our daily lives.
1. Rusting of Iron (Corrosion):
One of the most common and visually noticeable single displacement reactions is the rusting of iron. Iron (Fe) reacts with oxygen (O<sub>2</sub>) and water (H<sub>2</sub>O) in the air to form iron(III) oxide (Fe<sub>2</sub>O<sub>3</sub>·H<sub>2</sub>O), commonly known as rust.
4Fe(s) + 3O<sub>2</sub>(g) + 6H<sub>2</sub>O(l) → 4Fe(OH)<sub>3</sub>(s)
This reaction is a classic example of oxidation, where iron loses electrons to oxygen. The iron is displaced from its metallic state and forms a new compound, iron(III) hydroxide, which further dehydrates to form rust. This reaction is a significant concern in infrastructure and manufacturing, leading to significant economic losses due to material degradation.
2. The Reactivity of Metals with Acids:
Many metals react with acids to produce hydrogen gas and a salt. This is a quintessential single displacement reaction. For example, the reaction between zinc (Zn) and hydrochloric acid (HCl) produces zinc chloride (ZnCl<sub>2</sub>) and hydrogen gas (H<sub>2</sub>).
Zn(s) + 2HCl(aq) → ZnCl<sub>2</sub>(aq) + H<sub>2</sub>(g)
This reaction is utilized in various laboratory settings to produce hydrogen gas. The reactivity of the metal determines the vigor of the reaction; more reactive metals react more violently. This principle underscores the importance of safety precautions when handling such reactions.
3. Silver Tarnish:
The tarnishing of silver is another everyday example. Silver (Ag) reacts with hydrogen sulfide (H<sub>2</sub>S) in the air, often present in low concentrations, to form silver sulfide (Ag<sub>2</sub>S), the dark coating responsible for the tarnish.
2Ag(s) + H<sub>2</sub>S(g) → Ag<sub>2</sub>S(s) + H<sub>2</sub>(g)
This reaction demonstrates the displacement of hydrogen by silver, resulting in the formation of a less reactive and visually noticeable compound, silver sulfide. This tarnishing process highlights the reactivity of silver with certain sulfur compounds in the atmosphere.
4. Extraction of Metals from their Ores:
In the metallurgical industry, single displacement reactions are employed to extract metals from their ores. For instance, the extraction of iron from its oxide ores often involves the reduction of iron(III) oxide using carbon monoxide (CO).
Fe<sub>2</sub>O<sub>3</sub>(s) + 3CO(g) → 2Fe(l) + 3CO<sub>2</sub>(g)
This is a complex process, but at its core, carbon monoxide displaces iron from its oxide, resulting in the production of molten iron. This principle underlies many industrial processes crucial for obtaining various metals from their natural sources.
5. Production of Hydrogen Gas:
Besides the acid reaction mentioned earlier, hydrogen gas can also be produced via single displacement reactions involving water. Highly reactive metals like sodium (Na) and potassium (K) readily react with water to displace hydrogen.
2Na(s) + 2H<sub>2</sub>O(l) → 2NaOH(aq) + H<sub>2</sub>(g)
These reactions are highly exothermic and can be quite dangerous due to the explosive nature of hydrogen gas. However, the principle of hydrogen displacement from water is a crucial concept in understanding the reactivity of alkali metals.
6. Halide Reactions:
Halogens, such as chlorine (Cl<sub>2</sub>), bromine (Br<sub>2</sub>), and iodine (I<sub>2</sub>), can participate in single displacement reactions. A more reactive halogen can displace a less reactive halogen from its halide salt. For instance, chlorine can displace bromine from potassium bromide.
Cl<sub>2</sub>(g) + 2KBr(aq) → 2KCl(aq) + Br<sub>2</sub>(l)
This reaction demonstrates the relative reactivity of halogens, with chlorine being more reactive than bromine. The reaction sequence within the halogen group follows the order F<sub>2</sub> > Cl<sub>2</sub> > Br<sub>2</sub> > I<sub>2</sub>.
7. Photography Development:
The development of black and white photographic film involves single displacement reactions. Silver halide crystals in the film are exposed to light, triggering a reduction reaction. This converts silver ions (Ag<sup>+</sup>) to metallic silver (Ag), creating a latent image. While the exact chemical process is complex and involves multiple reactions, the reduction of silver ions to metallic silver is a key single displacement step.
8. Electroplating:
Electroplating is a process where a thin layer of metal is deposited onto another metal surface. This process utilizes single displacement reactions driven by an external electric current. For example, electroplating silver onto copper involves the reduction of silver ions (Ag<sup>+</sup>) from a silver salt solution onto the copper surface, displacing copper ions into the solution.
9. Bleaching Agents:
Many bleaching agents, like sodium hypochlorite (NaClO) in household bleach, work through single displacement reactions. The hypochlorite ion (ClO<sup>-</sup>) oxidizes colored compounds, effectively bleaching them. This oxidation often involves the displacement of electrons from the colored compound to the hypochlorite ion.
10. Biochemical Reactions:
Single displacement reactions are also fundamental to many biochemical processes. Enzyme-catalyzed reactions frequently involve the displacement of a functional group from one molecule to another.
Applications and Implications
Understanding single displacement reactions has numerous implications across various fields:
- Industrial Processes: These reactions are central to various industrial processes, including metal extraction, chemical synthesis, and manufacturing of various materials.
- Environmental Chemistry: Single displacement reactions play a role in environmental processes like corrosion, water pollution, and atmospheric chemistry.
- Analytical Chemistry: These reactions are used extensively in analytical chemistry for qualitative and quantitative analysis of substances.
- Medicine and Biotechnology: Single displacement reactions are involved in various biological processes and drug development.
Conclusion
Single displacement reactions are ubiquitous in our daily lives, often hidden within seemingly ordinary phenomena. From the rust on a discarded metal object to the development of a photograph, these reactions play a significant role in shaping our world. Understanding their underlying principles allows us to appreciate the chemical processes constantly occurring around us and highlights their importance in various aspects of science and technology. By recognizing and understanding these reactions, we can better appreciate the intricate chemical landscape that surrounds us daily. This understanding not only expands our scientific knowledge but also empowers us to make informed decisions regarding material selection, environmental impact, and the utilization of chemical processes in our daily lives.
Latest Posts
Latest Posts
-
What Is 64 Inches In Feet
Mar 19, 2025
-
How Do I Find The Number Of Neutrons
Mar 19, 2025
-
What Is 37 C In Fahrenheit
Mar 19, 2025
-
63 Kilograms Is How Many Pounds
Mar 19, 2025
-
Ones Working With Meters And Feet
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Single Displacement Reaction Examples In Everyday Life . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
