How Do I Find The Number Of Neutrons
Juapaving
Mar 19, 2025 · 6 min read
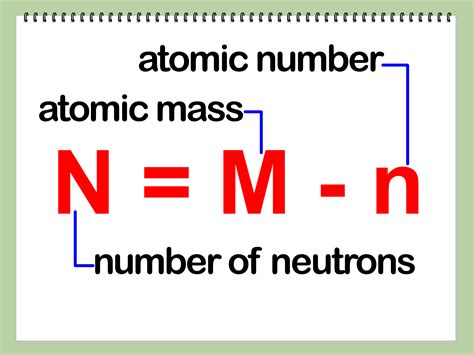
Table of Contents
How Do I Find the Number of Neutrons? A Comprehensive Guide
Determining the number of neutrons in an atom might seem like a complex task, but with the right understanding of atomic structure and a few simple calculations, it becomes straightforward. This comprehensive guide will walk you through various methods, explaining the concepts behind them and providing examples to solidify your understanding.
Understanding Atomic Structure: The Key to Neutron Calculation
Before diving into the calculations, let's refresh our understanding of atomic structure. An atom consists of three fundamental subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus.
- Neutrons: Neutral particles (no charge) also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells.
The number of protons defines the element. For example, all hydrogen atoms have one proton, all helium atoms have two, and so on. This number is known as the atomic number (Z) and is crucial for determining the number of neutrons.
The total number of protons and neutrons in an atom's nucleus is called the mass number (A). This number represents the approximate mass of the atom, as the mass of electrons is negligible compared to protons and neutrons.
Calculating the Number of Neutrons: The Formula and its Application
The key to finding the number of neutrons lies in the relationship between the mass number (A), the atomic number (Z), and the number of neutrons (N):
A = Z + N
Therefore, to find the number of neutrons (N), we can rearrange the formula:
N = A - Z
This simple equation is the cornerstone of neutron calculation. Let's illustrate this with some examples:
Example 1: Carbon-12
Carbon-12 (¹²C) is a common isotope of carbon. The superscript "12" represents its mass number (A = 12). The atomic number of carbon (Z) is 6 (you can find this on the periodic table). Therefore:
N = A - Z = 12 - 6 = 6
Carbon-12 has 6 neutrons.
Example 2: Uranium-235
Uranium-235 (²³⁵U) is a crucial isotope in nuclear reactors. Its mass number (A) is 235. The atomic number of uranium (Z) is 92. Thus:
N = A - Z = 235 - 92 = 143
Uranium-235 has 143 neutrons.
Example 3: Isotopes and Neutron Variation
It's important to note that isotopes of the same element have the same number of protons but differ in the number of neutrons. For example, carbon has several isotopes, including carbon-12 (⁶C), carbon-13 (¹³C), and carbon-14 (¹⁴C). While all have 6 protons, they have 6, 7, and 8 neutrons, respectively. This difference in neutron number accounts for the variations in their mass and properties.
Beyond the Basics: Understanding Isotopes and Isotopic Abundance
The examples above highlighted the importance of specifying the isotope when calculating the number of neutrons. Different isotopes of the same element exist due to variations in their neutron count. The periodic table usually lists the average atomic mass, which is a weighted average of the masses of all naturally occurring isotopes of an element. This average mass reflects the relative abundance of each isotope.
To calculate the number of neutrons for a specific isotope, you must know its mass number (A) – this information is crucial and will usually be explicitly stated.
Isotopic Abundance and Average Atomic Mass
The average atomic mass listed on the periodic table is a weighted average considering the abundance of each isotope. For instance, chlorine has two main isotopes: ³⁵Cl (75.77% abundance) and ³⁷Cl (24.23% abundance). To calculate the average atomic mass, you would use the following formula:
Average atomic mass = (mass of isotope 1 × abundance of isotope 1) + (mass of isotope 2 × abundance of isotope 2) + ...
This calculation doesn't directly give you the number of neutrons in a specific atom, but it’s essential for understanding the concept of isotopic abundance and its impact on the average atomic mass seen on the periodic table.
Advanced Concepts: Nuclear Stability and Neutron-Proton Ratio
The number of neutrons in an atom significantly influences its nuclear stability. The ratio of neutrons to protons (N/Z ratio) plays a crucial role in determining whether an atom is stable or radioactive.
-
Light elements (low atomic number): These elements tend to have a neutron-to-proton ratio close to 1:1 for stability.
-
Heavier elements (high atomic number): These elements require a higher neutron-to-proton ratio for stability. The excess neutrons help to overcome the repulsive forces between the increasing number of protons in the nucleus.
An imbalance in the N/Z ratio often leads to radioactive decay, where the unstable nucleus attempts to achieve a more stable configuration by emitting particles or energy.
Applications and Importance of Neutron Calculation
Understanding how to calculate the number of neutrons is vital in various fields:
-
Nuclear Physics: Neutron calculations are fundamental in understanding nuclear reactions, nuclear stability, and the behavior of radioactive isotopes.
-
Nuclear Medicine: Many medical diagnostic and therapeutic techniques rely on radioactive isotopes, and understanding their neutron composition is crucial for their safe and effective use.
-
Chemistry: Neutron numbers influence the chemical properties of isotopes, particularly in isotopic labeling and tracing experiments.
-
Material Science: The neutron count affects the properties of materials, such as their density, reactivity, and mechanical strength.
Troubleshooting Common Challenges
While the basic calculation is straightforward, some common challenges might arise:
-
Identifying the mass number: Ensure you correctly identify the mass number (A) of the isotope in question. The mass number is often written as a superscript before the element symbol (e.g., ¹⁴C).
-
Finding the atomic number: The atomic number (Z) can be easily found on the periodic table. Ensure you are using the correct atomic number for the specified element.
-
Dealing with average atomic mass: Remember that the average atomic mass on the periodic table is a weighted average of isotopes. It's not directly applicable for calculating neutrons in a specific isotope. You'll need the mass number of the specific isotope.
Conclusion: Mastering Neutron Calculation
Calculating the number of neutrons in an atom is a fundamental concept in chemistry and physics. By understanding the relationship between the mass number, atomic number, and number of neutrons, and by carefully considering isotopic variations, you can accurately determine the neutron count for any given atom. This knowledge is essential for comprehending atomic structure, nuclear stability, and the behavior of isotopes in various scientific applications. This comprehensive guide equips you with the necessary tools and knowledge to confidently tackle neutron calculations and further your understanding of the atomic world. Remember to always double-check your values and utilize reliable sources for your atomic numbers and mass numbers to ensure accurate calculations.
Latest Posts
Latest Posts
-
5 Letter Words Beginning With Ps
May 09, 2025
-
How Do You Write 3 5 As A Percentage
May 09, 2025
-
What Happens When Light Goes Through A Prism
May 09, 2025
-
What Is The Importance Of The Start And Stop Codons
May 09, 2025
-
Images And Names Of Musical Instruments
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Do I Find The Number Of Neutrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.