Region Of High Probability Of Finding An Electron
Juapaving
Mar 23, 2025 · 5 min read
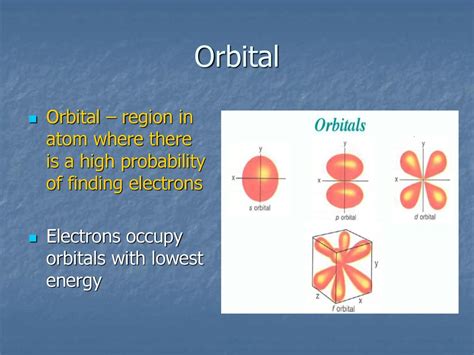
Table of Contents
Regions of High Probability of Finding an Electron: Unveiling the Quantum World
The seemingly simple question, "Where is an electron located?", unravels into a complex tapestry of quantum mechanics. Unlike classical mechanics where we can pinpoint the exact position of a particle, the behavior of electrons is governed by probabilities. Instead of precise locations, we talk about regions of high probability where an electron is likely to be found. This concept is fundamental to understanding the structure of atoms, molecules, and materials. This article delves deep into this fascinating aspect of quantum mechanics, exploring various concepts and their implications.
Understanding the Uncertainty Principle
The cornerstone of understanding electron probability is Heisenberg's Uncertainty Principle. This principle states that it's impossible to simultaneously know both the position and momentum of an electron with perfect accuracy. The more precisely we know one, the less precisely we know the other. This inherent uncertainty dictates that we cannot speak of an electron's exact location but rather its probability of being in a specific region.
Implications of the Uncertainty Principle for Electron Location
The Uncertainty Principle doesn't imply a lack of knowledge; rather, it highlights a fundamental limitation in our ability to describe the electron's behavior. It means that the electron doesn't possess a definite position until we measure it, and the act of measurement inherently disturbs its state. This is not a flaw in our measuring instruments, but a fundamental property of the quantum world.
Atomic Orbitals: Probability Density Maps
Instead of orbits as envisioned in the Bohr model, electrons are described by atomic orbitals. These orbitals are mathematical functions that represent the probability density of finding an electron at a particular point in space. The square of the wave function (Ψ²) gives the probability density, indicating the likelihood of finding an electron within a specific volume.
Visualizing Atomic Orbitals
Atomic orbitals are often depicted as three-dimensional shapes, representing regions of space where the probability of finding an electron is high. These visualizations are not depictions of the electron's trajectory, but rather probability distributions.
-
s orbitals: These are spherically symmetric, meaning the probability of finding the electron is the same in all directions at a given distance from the nucleus. The 1s orbital, the lowest energy level, has the highest probability density closest to the nucleus.
-
p orbitals: These orbitals are dumbbell-shaped, with two lobes of high probability density on either side of the nucleus. There are three p orbitals (px, py, pz) oriented along the x, y, and z axes, respectively.
-
d orbitals: These orbitals have more complex shapes, with four lobes (except for one dz² orbital with two lobes and a donut shape).
-
f orbitals: These orbitals possess even more intricate shapes and are found in higher energy levels.
Factors Influencing Electron Probability Density
Several factors influence the probability density of finding an electron in a particular region:
1. Principal Quantum Number (n)
This number determines the energy level and size of the orbital. Higher values of 'n' correspond to larger orbitals and higher energy levels, meaning the electron is likely to be found further from the nucleus. For instance, a 3s orbital is larger than a 2s orbital.
2. Azimuthal Quantum Number (l)
This number defines the shape of the orbital (s, p, d, f) and is related to the orbital angular momentum. Different 'l' values for the same 'n' correspond to different orbital shapes within the same energy level (e.g., 2s and 2p orbitals).
3. Magnetic Quantum Number (ml)
This number specifies the orientation of the orbital in space. For example, the three p orbitals (px, py, pz) have different ml values, indicating their orientation along different axes.
4. Spin Quantum Number (ms)
This number describes the intrinsic angular momentum (spin) of the electron. It doesn't directly influence the spatial probability distribution but plays a crucial role in determining the electron configuration and chemical behavior of atoms.
Electron Probability Density and Chemical Bonding
The concept of regions of high probability is crucial in understanding chemical bonding. When atoms interact, it is the overlap of atomic orbitals – regions of high electron probability – that leads to the formation of molecular orbitals and chemical bonds.
Covalent Bonding
In covalent bonding, atoms share electrons, resulting in a build-up of electron probability density between the nuclei. This shared electron probability density holds the atoms together, forming a stable molecule. The strength of the bond is related to the extent of orbital overlap and the resulting electron probability density in the bonding region.
Ionic Bonding
In ionic bonding, one atom loses an electron(s), and another gains it(s). The resulting ions are held together by electrostatic forces. The probability density of the transferred electron is significantly higher around the atom that gained it, contributing to the stability of the ionic bond.
Advanced Concepts
Beyond the basic atomic orbitals, more sophisticated concepts help us refine our understanding of electron probability:
Radial Probability Distribution
This function describes the probability of finding an electron at a particular distance from the nucleus, irrespective of direction. It shows that, for instance, the probability of finding a 1s electron is highest near the nucleus but doesn't drop sharply to zero even at larger distances.
Electron Density Mapping
Advanced techniques like X-ray crystallography and computational methods enable us to map the electron density in molecules and solids. These maps provide detailed information about the regions of high electron probability and help visualize chemical bonding and molecular structures.
Conclusion
The concept of regions of high probability of finding an electron is a cornerstone of quantum mechanics. While we cannot pinpoint an electron's exact location, the probability density functions provide a powerful framework for understanding atomic and molecular structure, chemical bonding, and the properties of materials. The probabilistic nature of electron location is not a limitation of our knowledge, but a fundamental aspect of the quantum world, revealing the beauty and complexity of nature at the atomic level. Understanding these probability distributions is key to unlocking a deeper understanding of chemistry, physics, and material science, paving the way for advancements in various fields. Continued research and technological advancements will further refine our ability to explore and visualize these regions of high probability, unveiling even more profound insights into the quantum world.
Latest Posts
Latest Posts
-
Why Is Earth Called The Blue Planet
Mar 24, 2025
-
What Is The Latent Heat Of Fusion
Mar 24, 2025
-
Earthworms And Leeches Belong To The Phylum
Mar 24, 2025
-
Classify The Below Solids As Amorphous Or Crystalline
Mar 24, 2025
-
Function Of Seminal Receptacles In Earthworm
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Region Of High Probability Of Finding An Electron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
