Pxidation Number Of H In H20
Juapaving
Apr 01, 2025 · 5 min read
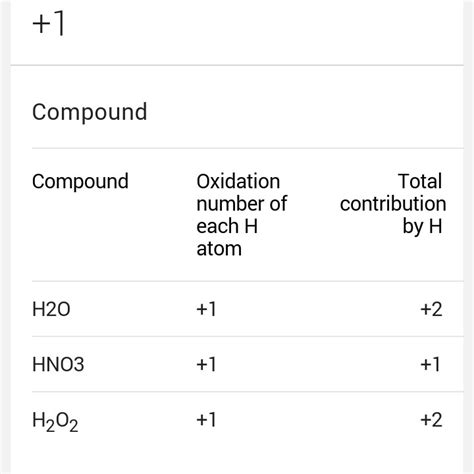
Table of Contents
Oxidation Number of H in H₂O: A Deep Dive
The seemingly simple question of the oxidation number of hydrogen in water (H₂O) opens a door to a deeper understanding of fundamental chemistry concepts. While many might immediately answer "+1," a nuanced exploration reveals the subtleties and contextual dependencies involved in assigning oxidation numbers. This comprehensive article delves into the intricacies of this seemingly straightforward topic, exploring its theoretical basis, practical applications, and exceptions.
Understanding Oxidation Numbers
Before focusing on H₂O, let's establish a firm grasp of oxidation numbers themselves. Oxidation numbers, also known as oxidation states, are integers assigned to atoms in a molecule or ion that represent the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic. This is a crucial point: oxidation numbers are not actual charges; they're a bookkeeping system to track electron transfer in chemical reactions.
Key Rules for Assigning Oxidation Numbers:
- Free elements: The oxidation number of an atom in its elemental form is always 0 (e.g., O₂ has an oxidation number of 0 for each oxygen atom).
- Monatomic ions: The oxidation number of a monatomic ion is equal to its charge (e.g., Na⁺ has an oxidation number of +1).
- Fluorine: Fluorine, the most electronegative element, always has an oxidation number of -1 in its compounds.
- Oxygen: Oxygen usually has an oxidation number of -2 in its compounds, except in peroxides (e.g., H₂O₂) where it's -1 and in compounds with fluorine where it's positive.
- Hydrogen: Hydrogen usually has an oxidation number of +1 in its compounds, except in metal hydrides (e.g., NaH) where it's -1.
- The sum of oxidation numbers: In a neutral molecule, the sum of the oxidation numbers of all atoms must equal zero. In a polyatomic ion, the sum must equal the charge of the ion.
Determining the Oxidation Number of H in H₂O
Applying these rules to H₂O, we can determine the oxidation number of hydrogen. Oxygen, being more electronegative than hydrogen, typically has an oxidation number of -2. Since H₂O is a neutral molecule, the sum of the oxidation numbers must be zero. Let 'x' represent the oxidation number of hydrogen. We can set up the equation:
2x + (-2) = 0
Solving for x, we get:
2x = 2
x = +1
Therefore, the oxidation number of hydrogen in water is +1.
Exceptions and Nuances
While the +1 oxidation state for hydrogen in H₂O is the most common and generally accepted, it's crucial to acknowledge potential complexities and exceptions:
1. Covalent Bonding Nature: The assignment of oxidation numbers is based on the hypothetical complete ionic character of the bonds. In reality, the H-O bond in H₂O has significant covalent character. Electrons are shared between hydrogen and oxygen, not completely transferred. The oxidation number represents a simplification for tracking electron transfer in reactions.
2. Electronegativity Differences: The electronegativity difference between hydrogen and oxygen is significant, leading to a polarized bond. Oxygen attracts the shared electrons more strongly, resulting in a partial negative charge on oxygen and a partial positive charge on hydrogen. This partial charge contributes to the +1 oxidation state assignment.
3. Hydrogen in Metal Hydrides: As mentioned earlier, hydrogen exhibits an oxidation number of -1 in metal hydrides like NaH, LiH, and CaH₂. In these compounds, hydrogen is less electronegative than the metal, leading to the transfer of an electron from the metal to the hydrogen atom.
4. Hydrogen Bonding: Water's unique properties, stemming from extensive hydrogen bonding, are not directly reflected in the oxidation number assignment. Hydrogen bonding, a strong intermolecular force, affects physical properties like boiling point and surface tension but does not alter the oxidation number of hydrogen within the individual H₂O molecule.
Applications and Importance of Oxidation Numbers
Understanding oxidation numbers is crucial for various aspects of chemistry:
1. Balancing Redox Reactions: Oxidation numbers are essential for balancing redox (reduction-oxidation) reactions, which involve the transfer of electrons. By tracking changes in oxidation numbers, we can ensure that the number of electrons lost in oxidation equals the number of electrons gained in reduction.
2. Predicting Reaction Products: Oxidation numbers can help predict the likely products of redox reactions. For instance, knowing the oxidation states of reactants can indicate whether a reaction is likely to occur and what the oxidation states of the products will be.
3. Nomenclature: Oxidation numbers are sometimes incorporated into the naming of compounds, particularly for transition metal complexes where the metal can exhibit multiple oxidation states.
4. Electrochemistry: In electrochemistry, oxidation numbers are crucial for understanding electrode potentials and predicting the spontaneity of electrochemical reactions. They are essential in understanding processes like corrosion, battery operation, and electrolysis.
5. Organic Chemistry: While less frequently used in organic chemistry compared to inorganic chemistry, oxidation numbers are still useful in understanding oxidation and reduction reactions of organic compounds. They can help identify the sites of oxidation and reduction and track the electron flow during reactions.
Advanced Concepts and Further Exploration
The seemingly simple concept of oxidation numbers opens doors to more complex ideas:
1. Fractional Oxidation Numbers: In some cases, especially in complex compounds with multiple bonding arrangements, fractional oxidation numbers can be assigned. This simply reflects the average oxidation state of the particular element across the molecule.
2. Formal Charges vs. Oxidation Numbers: Formal charges and oxidation numbers are distinct concepts. Formal charges represent the hypothetical charge on an atom in a molecule if all electrons in bonds were shared equally, while oxidation numbers consider the electronegativity differences and hypothetical ionic character.
3. Advanced Redox Titrations: The principles of oxidation numbers are critical in understanding and performing various types of redox titrations, allowing precise quantitative determination of the concentration of analytes.
Conclusion
The oxidation number of hydrogen in H₂O is fundamentally +1, reflecting the electronegativity difference between hydrogen and oxygen. However, a thorough understanding requires acknowledging the limitations of the model and considering the covalent nature of the bond. This seemingly straightforward calculation underscores the importance of mastering fundamental chemical concepts and recognizing their nuances. A deep dive into oxidation numbers opens avenues for understanding complex chemical reactions, balancing equations, predicting reaction outcomes, and exploring the broader world of redox chemistry. By applying these principles, we can effectively utilize this powerful tool in our exploration and application of chemistry.
Latest Posts
Latest Posts
-
Largest Cell Of The Human Body
Apr 02, 2025
-
What Is The Difference Between Solute And Solvent
Apr 02, 2025
-
How Big Is 7cm In Inches
Apr 02, 2025
-
What Is The Electron Configuration For Calcium
Apr 02, 2025
-
What Are The Chanses Of Getting Two
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Pxidation Number Of H In H20 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
