Ph Of Tap Water Vs Distilled Water
Juapaving
Mar 22, 2025 · 5 min read
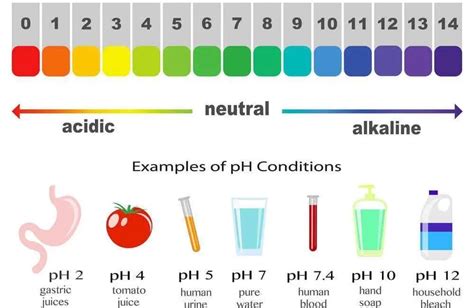
Table of Contents
pH of Tap Water vs. Distilled Water: A Comprehensive Comparison
Understanding the pH levels of different water types is crucial for various applications, from everyday drinking to industrial processes. This comprehensive guide delves into the specifics of tap water and distilled water, comparing their pH levels, factors influencing them, and the implications for various uses. We'll explore the reasons behind pH variations and how these variations can impact your health and other aspects of your life.
What is pH?
Before we dive into the comparison, let's establish a clear understanding of pH. pH is a measure of how acidic or basic (alkaline) a substance is. The scale ranges from 0 to 14, with 7 being neutral. A pH below 7 is acidic, while a pH above 7 is alkaline (basic). Each whole number change on the pH scale represents a tenfold change in acidity or alkalinity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5.
pH of Tap Water: A Variable Factor
The pH of tap water is highly variable and depends on several factors:
1. Source Water:
The original source of the water (river, lake, groundwater) significantly impacts its initial pH. Groundwater, for example, often has a higher mineral content, potentially influencing its pH.
2. Water Treatment Processes:
Municipal water treatment plants employ various processes to ensure water safety and potability. These processes can influence the pH:
- Chlorination: While primarily for disinfection, chlorination can subtly affect pH.
- pH Adjustment: Many plants adjust the pH of the water to an optimal range for corrosion control in the distribution system and to improve the effectiveness of disinfectants. This is frequently done by adding chemicals like lime or acid.
- Mineral Content: The presence of dissolved minerals like calcium and magnesium (hardness) can influence the pH.
3. Distribution System:
As water travels through the distribution pipes, interactions with the pipe materials (typically metal) can further alter its pH. Older pipes, especially those made of lead or iron, might contribute to increased mineral content and potential pH changes.
4. Geographic Location:
Tap water pH varies widely depending on geographic location due to differences in source water, soil composition, and water treatment practices.
Typical pH Range of Tap Water:
Generally, the pH of tap water falls within a range of 6.5 to 8.5. However, values outside this range are possible, especially in areas with unique geological conditions or water treatment protocols.
pH of Distilled Water: Near-Neutral Purity
Distilled water, on the other hand, undergoes a process of purification that significantly alters its pH. Distillation involves boiling water and collecting the condensed steam, leaving behind impurities, including minerals that affect pH. This process results in water that is considerably purer and closer to a neutral pH.
Typical pH Range of Distilled Water:
The pH of freshly distilled water is typically around 7.0, representing a neutral pH. However, exposure to air can lead to a slight increase in pH due to the absorption of carbon dioxide from the atmosphere, forming carbonic acid. This absorption typically results in a slight decrease in pH to a value between 5.0 and 6.0.
Comparing Tap Water and Distilled Water pH: Key Differences
The key difference lies in the variability: tap water exhibits a wide range of pH values based on various factors, while distilled water, in its purest form, starts at a nearly neutral pH of 7.0 and will slowly become slightly acidic due to carbon dioxide absorption.
| Feature | Tap Water | Distilled Water |
|---|---|---|
| pH Range | 6.5 - 8.5 (highly variable) | ~7.0 (initially); can decrease to 5.0-6.0 |
| Mineral Content | Variable, often higher | Very low |
| Purity | Lower, contains dissolved minerals and other substances | Higher, fewer impurities |
| Stability | Less stable, pH can fluctuate | More stable initially, then gradually decreases |
Implications of pH Differences
The differences in pH between tap and distilled water have implications for various applications:
1. Drinking Water:
While both are generally safe for drinking, the slightly acidic nature of distilled water might not appeal to everyone due to its taste. The pH of tap water is generally considered safe, but excessively high or low pH can indicate potential water quality issues.
2. Aquarium Use:
The pH of water is critical for aquatic life. Distilled water is often used as a base in aquariums, and its pH is adjusted to suit the specific needs of the fish species.
3. Industrial Applications:
Many industrial processes require water with specific pH levels. Distilled water's controlled pH makes it suitable for applications where impurities and pH variations could affect the process's outcome, such as in manufacturing, pharmaceuticals and laboratories.
4. Battery Life:
Distilled water is commonly used in lead-acid batteries to prevent corrosion and maintain their performance. Its consistent pH is beneficial for these applications.
5. Scientific Research:
In scientific experiments, especially those involving pH-sensitive reactions, distilled water is preferred due to its consistent and known pH.
Measuring pH: Simple Methods and Tools
Measuring the pH of water is straightforward using various methods:
- pH Test Strips: These inexpensive strips change color based on the pH level, providing a quick, visual estimate.
- Digital pH Meters: Electronic pH meters offer greater accuracy and precision compared to test strips. They measure the voltage difference between a pair of electrodes immersed in the water sample, thus determining the pH.
Importance of Accurate pH Measurement:
Accurate pH measurement is crucial for various applications, including water treatment, environmental monitoring, and scientific research. Inconsistent pH readings can lead to inaccurate assessments and potential problems.
Conclusion: Choosing the Right Water Based on pH Needs
Both tap water and distilled water serve different purposes, and their respective pH characteristics influence their suitability for various applications. Tap water, with its variable pH, is generally suitable for everyday drinking and many household uses. Distilled water, with its near-neutral and stable pH, is more appropriate for applications requiring high purity and consistent pH levels. Choosing the right type of water depends on understanding its characteristics and the specific requirements of the intended use. The choice will always depend on your needs and the importance of water purity for a given task. Accurate pH measurement is critical for making informed decisions about water quality and suitability across all applications.
Latest Posts
Latest Posts
-
Name Two Quadrilaterals That Have Four Right Angles
Mar 23, 2025
-
How Many Factors Does 21 Have
Mar 23, 2025
-
Ice Melting Physical Or Chemical Change
Mar 23, 2025
-
How Are Unicellular And Multicellular Alike
Mar 23, 2025
-
Which Statement About Viruses Is False
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Ph Of Tap Water Vs Distilled Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
