One Of The Physical Properties Of Bases Is That They-
Juapaving
Mar 15, 2025 · 6 min read
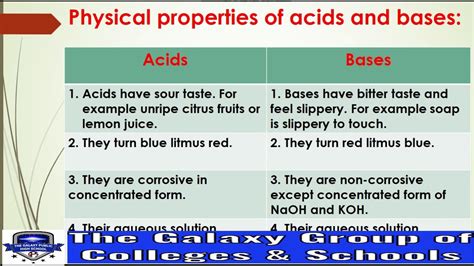
Table of Contents
One of the Physical Properties of Bases is That They… Feel Slippery! Understanding the Tactile and Other Physical Characteristics of Bases
One of the most striking – and perhaps unexpected – physical properties of bases is their slippery feel. This tactile characteristic, often described as soapy or slippery, is a key indicator of the presence of a base, especially in aqueous solutions. But the slippery sensation is just one facet of a broader range of physical properties that bases exhibit. Understanding these properties is crucial for safe handling, accurate identification, and effective application of bases in various fields, from everyday cleaning to advanced chemical processes.
The Slippery Sensation: A Closer Look
The soapy feel of bases isn't just a random quirk; it's a direct consequence of their chemical interaction with the skin. Bases, especially strong bases like sodium hydroxide (NaOH) or potassium hydroxide (KOH), react with the lipids (fats) and proteins in the skin's outermost layer, the stratum corneum. This reaction causes the saponification of fats – a process where fats are converted into soap. Soap, as we know, is a surfactant that lowers the surface tension of water, leading to the slippery sensation.
The Chemistry Behind the Slip
The saponification reaction involves the hydrolysis of esters (fats are esters of fatty acids and glycerol). The hydroxide ions (OH⁻) present in the base attack the ester bonds in the fats, breaking them down into glycerol and fatty acid salts. These salts are the soap molecules that contribute to the slippery feeling. This is why prolonged exposure to strong bases can cause significant skin irritation and damage. The breakdown of skin lipids disrupts the protective barrier, making the skin vulnerable to infections and further damage.
It's important to note: The slippery feel isn't unique to strong bases. Weak bases can also exhibit this property, though typically to a lesser extent. The intensity of the slippery sensation is directly related to the base's strength and concentration.
Other Key Physical Properties of Bases
Beyond the tactile sensation, several other physical properties help characterize and identify bases:
1. pH Value: A Defining Characteristic
The most fundamental property defining a base is its pH value. The pH scale measures the concentration of hydrogen ions (H⁺) in a solution. Bases have a pH greater than 7. Strong bases have a pH closer to 14, while weak bases have a pH closer to 7. pH measurement using indicators like litmus paper or pH meters is a crucial method for identifying and quantifying the basicity of a substance.
- Strong Bases: These completely dissociate in water, releasing a high concentration of hydroxide ions (OH⁻). Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)₂).
- Weak Bases: These only partially dissociate in water, releasing a lower concentration of hydroxide ions. Examples include ammonia (NH₃) and sodium carbonate (Na₂CO₃).
2. Taste: Bitter and Caustic
Another distinctive characteristic, though never to be tested directly, is the bitter taste associated with many bases. This bitter taste is a sensory warning signaling potential harm. Never taste any unknown chemical; this is a crucial safety precaution in any laboratory or chemical handling environment.
3. Reactivity with Acids: Neutralization Reactions
Bases react with acids in a process known as neutralization. This reaction leads to the formation of salt and water, effectively canceling out the acidic and basic properties. The reaction is exothermic, meaning it releases heat. This neutralization reaction is the basis of many titration techniques used in analytical chemistry to determine the concentration of an unknown acid or base.
The general equation for a neutralization reaction is:
Acid + Base → Salt + Water
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
4. Conductivity: Electrical Current Carriers
Aqueous solutions of bases conduct electricity because they contain ions (OH⁻ and the cation from the base). The ability to conduct electricity is directly proportional to the concentration of ions in the solution. Strong bases, with their complete dissociation into ions, are better conductors than weak bases. This property is used in conductivity meters to measure the concentration of ions and thus the strength of the base.
5. Appearance: Variable Physical States
Bases can exist in various physical states: solid, liquid, or gas. Many common bases, like sodium hydroxide and potassium hydroxide, are solid at room temperature. Ammonia is a gas at room temperature, while some bases exist as liquids. The physical state can influence handling and application methods.
Safety Precautions When Handling Bases
Because many bases are corrosive and can cause significant harm, appropriate safety precautions are crucial:
- Eye Protection: Always wear safety goggles or face shields when handling bases to protect against splashes.
- Protective Gloves: Use chemical-resistant gloves to prevent skin contact.
- Ventilation: Ensure adequate ventilation to minimize exposure to fumes or dust from bases.
- Neutralization: In case of spills, neutralize the base with a suitable acid, such as dilute acetic acid, following appropriate safety procedures.
- First Aid: In case of skin or eye contact, immediately flush the affected area with plenty of water for at least 15 minutes and seek medical attention.
Applications of Bases: A Wide Range of Uses
Bases have a wide array of applications across various industries:
- Cleaning Products: Many household cleaning products, including soaps, detergents, and drain cleaners, contain bases. Their ability to saponify fats and oils makes them effective at removing grease and dirt.
- Industrial Processes: Bases are used in numerous industrial processes, including the production of paper, textiles, and fertilizers. They play a critical role in chemical synthesis and catalysis.
- Food Industry: Some bases, like sodium bicarbonate (baking soda), are used as leavening agents in baking. They react with acids to produce carbon dioxide gas, which causes the dough to rise.
- Medicine: Bases are used in various pharmaceutical applications, including antacids to neutralize stomach acid and in the preparation of certain medicines.
Conclusion: Understanding the Importance of Base Properties
The physical properties of bases, from their slippery feel to their pH and reactivity, are essential for understanding their behavior and safe handling. The slippery sensation, while readily noticeable, is just one piece of the puzzle. Understanding the complete suite of physical properties—pH, reactivity with acids, conductivity, and appearance—is crucial for their safe and effective use across various scientific, industrial, and everyday applications. Remember that safety precautions should always be prioritized when working with bases, due to their potential corrosive nature. By understanding these properties and practicing safe handling procedures, we can harness the power and utility of bases while mitigating potential risks.
Latest Posts
Latest Posts
-
What Is 5 5 In Cm
Mar 15, 2025
-
The Horizontal Rows Of The Periodic Table Are Called
Mar 15, 2025
-
What Three Components Make Up A Nucleotide
Mar 15, 2025
-
What Is The Square Root Of 125
Mar 15, 2025
-
How Many Quarts Are In 2 Cubic Feet
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about One Of The Physical Properties Of Bases Is That They- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
