The Horizontal Rows Of The Periodic Table Are Called
Juapaving
Mar 15, 2025 · 7 min read
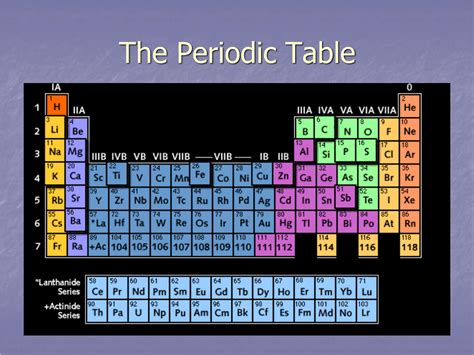
Table of Contents
The Horizontal Rows of the Periodic Table are Called Periods: A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its structure is crucial for comprehending chemical reactions and predicting the behavior of different elements. One of the fundamental aspects of this organization is the arrangement of elements into horizontal rows, known as periods. This article delves deep into the concept of periods, exploring their significance, the trends they reveal, and the underlying reasons for these observed patterns.
What are Periods in the Periodic Table?
The horizontal rows in the periodic table are called periods. Each period represents a principal energy level or shell in an atom. As we move across a period from left to right, the atomic number increases, meaning the number of protons and electrons in the atom increases. This increase in electrons leads to significant changes in the element's chemical and physical properties. Crucially, elements within the same period have the same number of electron shells.
Number of Periods:
Currently, the periodic table consists of seven periods. The number of elements in each period varies; it's not a fixed number. This variation is a direct consequence of the filling of electron subshells within each energy level.
- Period 1: Contains only two elements, hydrogen (H) and helium (He), as it only has the 1s subshell.
- Period 2: Has eight elements, filling the 2s and 2p subshells.
- Period 3: Also has eight elements, filling the 3s and 3p subshells.
- Period 4: Contains 18 elements, incorporating the filling of the 3d subshell along with the 4s and 4p subshells. This is where the transition metals begin to appear.
- Period 5: Similar to period 4, it houses 18 elements due to the filling of the 4d subshell and the 5s and 5p subshells.
- Period 6: Contains 32 elements, including the filling of the 4f subshell (lanthanides), the 5d subshell, and the 6s and 6p subshells.
- Period 7: Is currently incomplete, but will eventually encompass 32 elements, similarly including the filling of the 5f subshell (actinides), the 6d subshell, and the 7s and 7p subshells.
Periodic Trends: The Story Told by Periods
Moving across a period reveals fascinating patterns in the properties of elements. These patterns, known as periodic trends, are directly linked to the increasing nuclear charge and the gradual filling of electron shells. Understanding these trends is essential for predicting how elements will react and behave.
1. Atomic Radius: A Decreasing Trend
The atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period. This is because, while additional electrons are being added to the same energy level, the nuclear charge (number of protons) is also increasing. This stronger positive charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
2. Ionization Energy: An Increasing Trend
Ionization energy is the energy required to remove an electron from a gaseous atom. It generally increases across a period. This is directly related to the increasing nuclear charge. As the nuclear charge increases, the attraction between the nucleus and the outermost electrons strengthens, making it more difficult to remove an electron, thus requiring more energy.
3. Electronegativity: A Growing Attraction
Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. It generally increases across a period. Again, the increasing nuclear charge is the culprit. A higher nuclear charge exerts a greater pull on bonding electrons, increasing the atom's electronegativity.
4. Electron Affinity: The Energy Change Upon Electron Addition
Electron affinity is the energy change that occurs when an atom gains an electron. While there are some exceptions, there's a general trend of increasing electron affinity across a period. Atoms with higher nuclear charges generally have a greater tendency to attract and hold an additional electron, releasing energy in the process.
5. Metallic Character: A Gradual Transition
Metallic character, encompassing properties like conductivity, malleability, and ductility, generally decreases across a period. Elements on the left side of a period tend to be highly metallic, while elements on the right tend to be non-metallic. This change reflects the increasing tendency for atoms to gain electrons rather than lose them as one moves across the period.
The Significance of Periods in Chemical Reactivity
The position of an element within a period significantly influences its chemical reactivity. For instance:
- Alkali Metals (Group 1): Located at the beginning of each period (except period 1), these elements have one valence electron, making them highly reactive. They readily lose this electron to achieve a stable electron configuration.
- Halogens (Group 17): Located towards the end of each period, these elements have seven valence electrons. They readily gain one electron to achieve a stable octet, making them also highly reactive.
- Noble Gases (Group 18): Located at the end of each period, these elements have a complete outermost electron shell (except helium, which has a complete 1s subshell). This stable configuration makes them very unreactive, often referred to as inert.
Exceptions to the Trends: A Deeper Look
While the periodic trends discussed above are generally observed, there are exceptions. These exceptions arise from the complex interplay of electron-electron repulsions, shielding effects, and the specific orbital shapes involved. For example:
- Irregularities in Ionization Energy: There are instances where the ionization energy doesn't strictly increase across a period. This can be attributed to the stability associated with half-filled or fully filled subshells. For example, the ionization energy of nitrogen is higher than oxygen, despite oxygen having a higher nuclear charge. This is because nitrogen has a half-filled p subshell (p³ configuration), a relatively stable arrangement.
- Variations in Atomic Radius: The decrease in atomic radius across a period isn't strictly linear. Subtle variations can be observed due to the shielding effects of inner electrons and electron-electron repulsions.
- Electron Affinity Anomalies: Similar to ionization energy, electron affinity does not always follow a perfectly increasing trend. The energy released upon the addition of an electron is affected by the electron-electron repulsions in the already-present electron cloud.
Beyond the Basic Trends: Exploring Deeper Connections
Understanding periodic trends provides a foundational understanding of chemical behavior. However, the story extends far beyond these fundamental patterns. Further exploration reveals nuanced connections between period position and various other properties:
- Melting and Boiling Points: These properties are not directly and consistently linked to the period number alone, but show relationships influenced by the type of bonding present (metallic, covalent, ionic), atomic size, and intermolecular forces.
- Density: Density also doesn't follow a simple trend across periods. It is a complex function of atomic mass and atomic volume.
- Magnetic Properties: The presence of unpaired electrons influences magnetic properties. Transition metals, often found in the middle of periods, often exhibit paramagnetism or ferromagnetism due to the presence of unpaired electrons in d orbitals.
Conclusion: Periods – The Key to Unlocking Chemical Understanding
The horizontal rows, or periods, of the periodic table are not simply a way to organize elements; they reveal fundamental patterns in atomic structure and chemical behavior. By understanding the trends observed across periods – the decrease in atomic radius, the increase in ionization energy and electronegativity, and the changes in metallic character – we gain powerful insights into the reactivity and properties of various elements. While exceptions exist, the overarching trends provide a framework for predicting and understanding the rich and complex world of chemical interactions. Furthermore, the depth of understanding available through studying periods expands our ability to predict novel chemical behavior and contribute to advancements in various scientific fields. The periodic table, with its periods as a cornerstone, remains a powerful tool for scientists and a testament to the elegance of the organization of matter.
Latest Posts
Latest Posts
-
Identify The Equivalent Expression For Each Of The Expressions Below
Mar 15, 2025
-
What Is The Common Multiple Of 9 And 12
Mar 15, 2025
-
Organisms That Make Their Own Food Are Called
Mar 15, 2025
-
The Process Of Grouping Things Based On Their Common Characteristics
Mar 15, 2025
-
What Is The Least Common Multiple Of 3 And 9
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about The Horizontal Rows Of The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
