On Fahrenheit Scale Water Boils At
Juapaving
Mar 18, 2025 · 6 min read
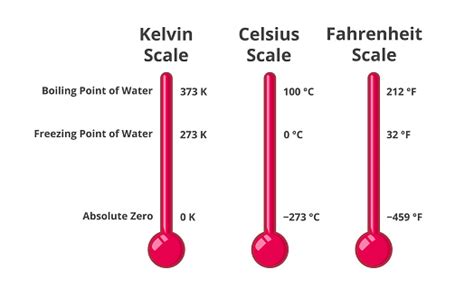
Table of Contents
On the Fahrenheit Scale, Water Boils At: A Deep Dive into Temperature Measurement
The seemingly simple question, "On the Fahrenheit scale, water boils at...?" holds a wealth of information about the history of temperature measurement, the properties of water, and the intricacies of scientific scales. The answer, of course, is 212 degrees Fahrenheit (212°F), but understanding why this is so requires a journey into the fascinating world of thermometry.
The Genesis of Fahrenheit: A Historical Perspective
Unlike the Celsius scale, which is based on the freezing and boiling points of water, the Fahrenheit scale's origins are more nuanced and somewhat arbitrary. Developed by the German physicist Daniel Gabriel Fahrenheit in the early 18th century, the scale initially relied on a few reference points:
- 0°F: Originally defined as the temperature of a mixture of ice, water, and ammonium chloride (a freezing mixture). This point represented the lowest temperature Fahrenheit could consistently achieve in his laboratory using this mixture.
- 32°F: The freezing point of water.
- 96°F: Initially designated as the average human body temperature.
Fahrenheit's choice of these reference points was influenced by his experimentation and the technology available at the time. It's crucial to note that human body temperature can fluctuate, so this reference point is not as precisely defined as the freezing point of water.
Over time, the definition of the Fahrenheit scale has been refined for greater accuracy. Today, it is officially defined in relation to the Celsius scale:
- 0°C = 32°F (Freezing point of water)
- 100°C = 212°F (Boiling point of water)
This relationship allows for seamless conversion between the two scales, highlighting the importance of standardized measurements in science and engineering.
Why 212°F? The Significance of Water's Boiling Point
The boiling point of water at 212°F is not merely a convenient number; it is a fundamental physical property of water. This temperature represents the point at which the vapor pressure of water equals the surrounding atmospheric pressure. In simpler terms:
- Vapor Pressure: The pressure exerted by water molecules as they transition from liquid to gas (vapor).
- Atmospheric Pressure: The pressure exerted by the weight of the atmosphere on the Earth's surface.
At sea level, standard atmospheric pressure is approximately 1 atmosphere (atm). When the vapor pressure of water reaches this level, the water begins to boil, transitioning rapidly from liquid to gaseous state (steam).
It's important to remember that the boiling point of water is not a constant. It varies with changes in atmospheric pressure. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, such as in a pressure cooker, water boils at a higher temperature. This explains why cooking times can differ significantly at various altitudes.
Implications of the Boiling Point: Practical Applications
The boiling point of water at 212°F has profound implications in various aspects of our lives, from cooking and cleaning to industrial processes:
- Cooking: Boiling water is fundamental to many cooking techniques, from pasta and vegetables to sterilization. The consistent boiling point ensures predictable cooking times and food safety.
- Cleaning: High-temperature boiling water is an effective disinfectant, killing harmful bacteria and viruses. Steam cleaning utilizes this principle for effective cleaning and sanitization.
- Industrial Processes: Many industrial processes rely on boiling water for heating, sterilization, and chemical reactions. The precisely defined boiling point ensures process consistency and efficiency.
- Steam Power: The generation of steam from boiling water is a crucial element in steam turbines, used for electricity generation and other applications. The high energy content of steam makes it an efficient power source.
- Scientific Experiments: The boiling point of water serves as a critical reference point in various scientific experiments, especially in calibrating and validating temperature-measuring instruments.
Understanding Temperature Scales: A Comparative Analysis
To fully appreciate the significance of 212°F, let's compare it with other common temperature scales:
- Celsius (Centigrade): This scale, also known as the centigrade scale, sets the freezing point of water at 0°C and the boiling point at 100°C. It's widely used in scientific contexts and most of the world for everyday purposes.
- Kelvin (Absolute): The Kelvin scale is an absolute temperature scale, meaning its zero point (0 K) represents absolute zero – the theoretical point at which all molecular motion ceases. The Kelvin scale is frequently used in scientific calculations and thermodynamics. Water boils at 373.15 K.
The conversion between Fahrenheit and Celsius is given by the following formulas:
- °C = (°F - 32) × 5/9
- °F = (°C × 9/5) + 32
Beyond the Boiling Point: Properties of Water at Different Temperatures
While the boiling point is crucial, it's vital to understand the properties of water at various temperatures:
- Below 0°C (32°F): Water freezes, transitioning into ice. The density of ice is lower than liquid water, which is why ice floats.
- Between 0°C (32°F) and 100°C (212°F): Water exists in its liquid state. Its properties like density, viscosity, and heat capacity change with temperature.
- Above 100°C (212°F): Water boils and turns into steam, a gaseous phase. Steam possesses significant thermal energy and can be used for various applications.
Factors Affecting the Boiling Point: Altitude and Pressure
As mentioned earlier, the boiling point of water is sensitive to changes in atmospheric pressure:
- Altitude: At higher altitudes, atmospheric pressure is lower. This means water requires less energy to overcome the external pressure and boils at a lower temperature. For example, at high mountain altitudes, water might boil at temperatures significantly below 212°F.
- Pressure: In a pressure cooker, increased pressure raises the boiling point of water, allowing for faster cooking times due to the higher temperature. Conversely, reducing pressure, as in a vacuum, lowers the boiling point.
The Importance of Accurate Temperature Measurement
Accurate temperature measurement is crucial across numerous fields, from scientific research to industrial processes and everyday life. The consistency of the boiling point of water, at 212°F at standard atmospheric pressure, provides a reliable reference point for calibrating thermometers and ensuring accurate measurements.
Modern thermometers use a variety of principles for temperature measurement, including:
- Liquid-in-glass thermometers: These rely on the thermal expansion of a liquid, such as mercury or alcohol, to indicate temperature.
- Thermocouples: These measure temperature based on the voltage generated at the junction of two dissimilar metals.
- Resistance Temperature Detectors (RTDs): These utilize the change in electrical resistance of a metal with temperature.
- Infrared Thermometers: These measure temperature by detecting infrared radiation emitted by an object.
Each type of thermometer has its own advantages and limitations, making it suitable for different applications. The accuracy and precision of temperature measurement are paramount for reliable results in any context.
Conclusion: The Enduring Significance of 212°F
The seemingly simple answer, "On the Fahrenheit scale, water boils at 212°F," opens a window into a rich tapestry of scientific concepts, historical developments, and practical applications. Understanding the boiling point of water, its dependence on pressure and altitude, and its significance in various fields underscores the importance of precise measurement and the enduring relevance of this fundamental physical property. From cooking a meal to generating electricity, the 212°F mark on the Fahrenheit scale serves as a constant reminder of the power and precision inherent in scientific understanding. The seemingly simple question thus reveals a complex and fascinating world of scientific inquiry.
Latest Posts
Latest Posts
-
How To Find The Gcd Of Three Numbers
Mar 18, 2025
-
Examples Of Pull And Push Forces
Mar 18, 2025
-
What Is 180 Minutes In Hours
Mar 18, 2025
-
A Homogeneous Mixture Of Two Or More Substances Is A
Mar 18, 2025
-
3 By 3 System Of Equations Solver
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about On Fahrenheit Scale Water Boils At . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
