On A Solubility Curve Solids Are Sometimes Referred To As...
Juapaving
Mar 05, 2025 · 7 min read
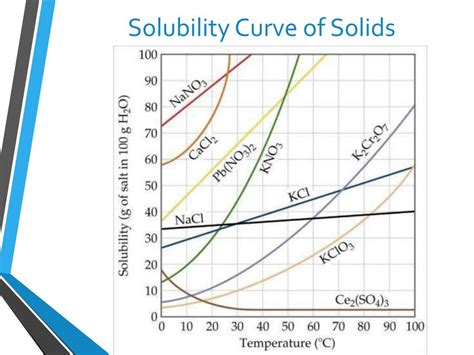
Table of Contents
On a Solubility Curve, Solids are Sometimes Referred to as… Saturated Solutions! Understanding Solubility and its Curves
Solubility, a fundamental concept in chemistry, describes the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure. Understanding solubility is crucial in numerous fields, from pharmaceuticals and environmental science to material science and geology. When we depict solubility graphically, we use solubility curves. On these curves, solids at their maximum dissolution point are often referred to as saturated solutions. Let's delve deeper into this fascinating concept.
What is a Solubility Curve?
A solubility curve is a graphical representation of the relationship between the solubility of a substance (usually a solid) and temperature. It shows how much of a solute can dissolve in a fixed amount of solvent (typically 100g of water) at different temperatures. These curves are typically constructed by experimentally determining the solubility of a solute at various temperatures and plotting the data on a graph. The x-axis represents temperature, and the y-axis represents solubility (usually expressed in grams of solute per 100g of water).
Key Features of Solubility Curves:
-
Positive Slope: Many solubility curves for solids show a positive slope, indicating that solubility increases with increasing temperature. This means that more solid can dissolve in the solvent as the temperature rises. This is because higher temperatures provide more kinetic energy to the solvent molecules, allowing them to more effectively break apart the solute particles and incorporate them into the solution.
-
Negative Slope: Some substances, however, exhibit a negative slope, meaning their solubility decreases as temperature increases. This is less common for solids but is observed in certain compounds.
-
Saturation Point: The curve itself represents the saturation point for the solute at each temperature. Any point on the curve represents a saturated solution – a solution containing the maximum amount of dissolved solute at that temperature.
-
Unsaturated and Supersaturated Solutions: Points below the curve represent unsaturated solutions, where less solute is dissolved than the maximum possible at that temperature. Points above the curve represent supersaturated solutions, which are unstable and contain more solute than can normally dissolve at that temperature. These are often created by carefully cooling a saturated solution without disturbing it, allowing it to retain more solute than it would normally hold at the lower temperature.
Why are Solids at Maximum Dissolution Referred to as Saturated Solutions?
When a solid reaches its maximum solubility in a solvent at a particular temperature, the solution is said to be saturated. This means that no more solute can dissolve in the solvent under those conditions. The rate of dissolution (the rate at which solid dissolves) equals the rate of crystallization (the rate at which dissolved solid comes out of solution). The system is in a dynamic equilibrium.
This dynamic equilibrium is a key characteristic of a saturated solution. While it appears static from the outside, at the molecular level, solute particles are constantly dissolving and crystallizing at the same rate. Adding more solute to a saturated solution will not increase the concentration of the dissolved solute; it will simply remain as undissolved solid at the bottom of the container.
Factors Affecting Solubility: Temperature and Pressure
Temperature is the most significant factor influencing the solubility of solids in liquids. As discussed earlier, increased kinetic energy at higher temperatures facilitates the dissolution process for most solids. However, pressure has a negligible effect on the solubility of solids in liquids, unlike gases.
Types of Solutions Based on Solubility: Unsaturated, Saturated, and Supersaturated
-
Unsaturated Solution: Contains less solute than it can dissolve at a given temperature. More solute can be added to an unsaturated solution and it will still dissolve.
-
Saturated Solution: Contains the maximum amount of solute that can dissolve at a given temperature. Adding more solute will not result in increased dissolution; excess solute will remain undissolved. This is where the term "saturated" directly relates to the solubility curve for solids.
-
Supersaturated Solution: Contains more solute than it can normally dissolve at a given temperature. This is a metastable state; it's unstable and prone to crystallization. A small disturbance, such as adding a seed crystal or scratching the container, can trigger rapid crystallization, resulting in the precipitation of excess solute.
Practical Applications of Solubility Curves and Saturated Solutions
The knowledge of solubility curves and the concept of saturation has numerous applications:
-
Pharmaceutical Industry: Solubility is critical in drug formulation. Understanding how much of a drug substance can dissolve in the body's fluids is vital for determining its bioavailability and effectiveness. Saturated solutions can be used to deliver a consistent amount of drug over time.
-
Environmental Science: Solubility studies are essential in understanding pollutant behavior in the environment. Knowing the solubility of pollutants helps in assessing their environmental impact and developing remediation strategies.
-
Material Science: Solubility is key in materials science for creating new materials with specific properties. Understanding the solubility of different components allows scientists to fine-tune the properties of alloys, polymers, and other materials.
-
Geology: Solubility plays a significant role in geological processes, such as the formation of caves and mineral deposits. The solubility of minerals in groundwater determines the composition of the water and the types of formations that can develop over time.
-
Food Science: Solubility is crucial in food science and technology. Understanding the solubility of various food components helps in developing food products with the desired textures and flavors.
-
Chemical Engineering: Solubility is central to many chemical processes, including crystallization, precipitation, and extraction. Engineers use solubility data to optimize the efficiency of these processes.
Beyond the Basics: Factors Affecting Solubility Beyond Temperature
While temperature is the dominant factor affecting the solubility of solids, other factors play a role:
-
Nature of the Solute and Solvent: The chemical nature of both the solute and the solvent significantly impacts solubility. "Like dissolves like" is a general rule: polar solvents tend to dissolve polar solutes, while nonpolar solvents tend to dissolve nonpolar solutes.
-
Pressure (Minor Effect for Solids): As mentioned before, pressure has a negligible impact on the solubility of solids. However, it plays a substantial role in the solubility of gases.
-
Presence of Other Ions: The presence of other ions in the solution can affect the solubility of a particular ion through the common-ion effect. The presence of a common ion reduces the solubility of the slightly soluble salt.
-
Particle Size: Smaller solute particles dissolve faster than larger particles, increasing the rate of dissolution, although it does not affect the ultimate solubility.
-
Stirring: Stirring increases the rate of dissolution by bringing fresh solvent into contact with the solute particles but does not change the equilibrium solubility.
Understanding Solubility Curves: A Deeper Dive into Interpretation
Analyzing solubility curves allows us to predict the behavior of solutions under various conditions. By understanding the slope of the curve, we can determine how temperature affects solubility. A steep slope indicates a significant change in solubility with temperature changes, while a shallow slope indicates a less dramatic change. The curve also allows us to identify the saturation point for the solute at different temperatures, which is crucial for many applications. Careful analysis of a solubility curve can provide invaluable insights into the interactions between the solute and solvent.
Conclusion: Solubility – A Cornerstone of Chemistry and Beyond
The solubility curve, and the concept of saturation it clearly depicts, is not merely a graphical representation; it's a powerful tool that reveals the intricate relationship between solute, solvent, and temperature. Understanding solubility curves and the concept of saturated solutions is crucial for advancements across numerous scientific and technological fields. From the development of life-saving pharmaceuticals to the understanding of environmental pollution and geological processes, the principles of solubility form a cornerstone of countless applications. The seemingly simple concept of a saturated solution, clearly illustrated on a solubility curve, unlocks a world of understanding and possibility.
Latest Posts
Latest Posts
-
What Type Of Medium Travels The Fastest
Mar 06, 2025
-
In Mechanism Photophosphorylation Is Most Similar To
Mar 06, 2025
-
What Is 3 10 As A Percent
Mar 06, 2025
-
How Many Chambers Does The Heart Of A Frog Have
Mar 06, 2025
-
What Is The Gcf Of 42 And 28
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about On A Solubility Curve Solids Are Sometimes Referred To As... . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
