Number Of Valence Electrons In Strontium
Juapaving
Mar 23, 2025 · 5 min read
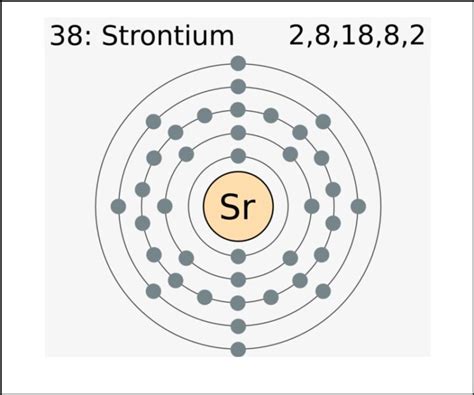
Table of Contents
The Number of Valence Electrons in Strontium: A Deep Dive
Strontium, a fascinating alkaline earth metal, plays a significant role in various applications, from fireworks to medical treatments. Understanding its electronic structure, particularly the number of valence electrons, is crucial to comprehending its chemical behavior and reactivity. This article delves into the intricacies of strontium's valence electrons, exploring its atomic structure, periodic table placement, and the implications of its valence electron configuration for its chemical properties and applications.
Understanding Valence Electrons
Before focusing specifically on strontium, let's establish a fundamental understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of chemical bonds it can form. The number of valence electrons directly influences an element's chemical properties and its position within the periodic table.
Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas (Group 18 elements). This tendency is described by the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve eight electrons in their outermost shell. Exceptions to the octet rule exist, particularly for elements in the transition metal series and beyond.
Strontium's Position in the Periodic Table
Strontium (Sr) is an alkaline earth metal located in Group 2 and Period 5 of the periodic table. Its atomic number is 38, meaning it has 38 protons and 38 electrons in a neutral atom. The periodic table's organization is not arbitrary; it reflects the underlying electronic structure of the elements. The group number provides crucial information about the number of valence electrons.
Group 2: The Alkaline Earth Metals
Group 2 elements, also known as alkaline earth metals, are characterized by having two valence electrons. This consistent valence electron count is a defining characteristic of the group, resulting in similar chemical properties across the group members. These elements tend to lose their two valence electrons to form 2+ ions, achieving a stable electron configuration.
Strontium's Electronic Configuration
To determine the number of valence electrons in strontium, we need to examine its electronic configuration. The electronic configuration describes the arrangement of electrons within an atom's energy levels and sublevels. For strontium, the electronic configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²
This configuration shows how the 38 electrons are distributed among the various energy levels and sublevels.
Identifying Valence Electrons in Strontium's Configuration
The outermost shell in strontium's electron configuration is the 5s shell, containing two electrons. Therefore, strontium has two valence electrons. The inner shells (1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p⁶) are considered core electrons and do not actively participate in chemical bonding.
Chemical Properties and Reactivity of Strontium
The presence of two valence electrons significantly influences strontium's chemical properties and reactivity. Due to its relatively low ionization energy, strontium readily loses these two electrons to form a Sr²⁺ ion, achieving a stable noble gas configuration similar to krypton. This tendency to lose electrons makes strontium highly reactive, especially with nonmetals like oxygen and halogens.
Reactions of Strontium
-
Reaction with Oxygen: Strontium reacts vigorously with oxygen in the air to form strontium oxide (SrO). This reaction is exothermic and produces a bright, reddish flame.
-
Reaction with Water: Strontium reacts slowly with cold water and more rapidly with hot water to produce strontium hydroxide (Sr(OH)₂) and hydrogen gas.
-
Reaction with Halogens: Strontium readily reacts with halogens (fluorine, chlorine, bromine, iodine) to form strontium halides (SrF₂, SrCl₂, SrBr₂, SrI₂). These reactions are also exothermic and can be quite vigorous.
-
Reaction with Acids: Strontium reacts with dilute acids, such as hydrochloric acid, to produce strontium salts and hydrogen gas.
Applications of Strontium
The unique properties stemming from its two valence electrons enable strontium to find applications in a variety of fields:
1. Fireworks and Pyrotechnics
Strontium's ability to emit a brilliant crimson red color when burned makes it an essential component in fireworks and pyrotechnics. The intense red light produced is a result of electronic transitions within the strontium ion (Sr²⁺) as it returns to a lower energy state after excitation.
2. Alloys
Strontium is used in various metal alloys to improve their properties. For example, it can enhance the strength and castability of aluminum alloys.
3. Medical Applications
Certain strontium isotopes are used in medical applications, particularly in bone scans and in the treatment of bone diseases like osteoporosis. Strontium ranelate, for example, is a medication used to increase bone density and reduce the risk of fractures. This use leverages strontium's chemical similarity to calcium, as both elements readily interact with bone tissue.
4. Other Applications
Strontium is used in various other niche applications, including:
- Phosphors: Strontium-containing compounds are used in phosphors for cathode ray tubes and fluorescent lamps.
- Ferrites: Strontium ferrites are used in permanent magnets.
- Ceramics: Strontium compounds are employed in certain types of ceramics.
Conclusion
Strontium, with its two valence electrons, exhibits characteristic properties of an alkaline earth metal. This relatively simple electronic configuration is the key to understanding its reactivity and its broad range of applications. Its tendency to lose these two electrons to form a 2+ ion governs its chemical interactions and explains its role in various industrial processes and technologies, from the vibrant colors in fireworks to the critical role it plays in medical treatments. Further research into strontium's properties and potential uses continues to expand its importance across diverse scientific and technological fields. The seemingly simple number of two valence electrons holds the key to understanding this element's complex and multifaceted impact.
Latest Posts
Latest Posts
-
Which Of The Following Is An Example Of Radiation
Mar 24, 2025
-
A Line That Intersects A Circle In Exactly One Point
Mar 24, 2025
-
Is Boiling An Endothermic Or Exothermic Process
Mar 24, 2025
-
What Is Resistance To Motion Called
Mar 24, 2025
-
Kirchhoffs Junction Rule Is A Statement Of
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Strontium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
