Number Of Valence Electrons In Iron
Juapaving
Mar 20, 2025 · 5 min read
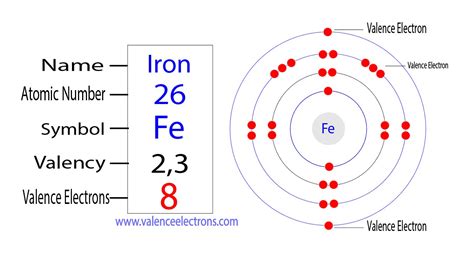
Table of Contents
Unveiling the Mysteries of Iron: Delving into its Valence Electrons
Iron, a ubiquitous element crucial to life and industry, holds a fascinating complexity within its seemingly simple atomic structure. Understanding its properties, particularly the number of valence electrons, is key to unlocking its diverse applications and its significant role in biological and chemical processes. This in-depth exploration will delve into the intricacies of iron's electronic configuration, explaining the concept of valence electrons, their determination, and the implications of iron's specific valence electron count.
What are Valence Electrons?
Before diving into the specifics of iron, let's establish a firm understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the electrons most involved in chemical bonding and interactions with other atoms. The number of valence electrons determines an element's chemical reactivity and the types of bonds it can form – ionic, covalent, or metallic. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often resembling that of a noble gas (group 18 elements). This stable configuration, often involving a full outer shell, is the driving force behind chemical bonding.
Determining the Number of Valence Electrons
The number of valence electrons can be determined in several ways. The most straightforward method is by examining an element's electron configuration. This configuration describes the arrangement of electrons in different energy levels or shells within the atom. Each energy level can hold a specific number of electrons, with the outermost shell containing the valence electrons. The periodic table is also a valuable tool. The group number (vertical column) of an element in the periodic table often indicates the number of valence electrons for the main group elements (s and p block elements). However, transition metals, like iron, exhibit more complex behavior.
Iron's Electronic Configuration and Valence Electrons
Iron (Fe) is a transition metal with an atomic number of 26, meaning it has 26 protons and 26 electrons in a neutral atom. Its electron configuration is: 1s²2s²2p⁶3s²3p⁶4s²3d⁶. This configuration indicates the distribution of electrons across various energy levels and sublevels.
The key to understanding iron's valence electrons lies in its outermost shells and the subtle nuances of transition metal behavior. While the 4s subshell is generally considered the outermost shell, transition metals often involve the (n-1)d subshell in bonding. This means both the 4s and 3d electrons can participate in chemical bonding, making the determination of valence electrons more complex than in main group elements.
Therefore, while the simple answer might seem to be two (from the 4s²), a more accurate representation considers the involvement of both 4s and 3d electrons, leading to a variable number of valence electrons for iron, typically ranging from 2 to 6. The exact number of valence electrons involved depends on the specific chemical context and the type of bond formed.
Why the Variable Valence Electrons?
The variable valence electron count of iron is a direct consequence of its electronic configuration and the relatively small energy difference between the 3d and 4s orbitals. This proximity in energy allows electrons from both orbitals to participate in chemical bonding, resulting in different oxidation states. Iron commonly displays oxidation states of +2 (ferrous) and +3 (ferric), reflecting the different numbers of valence electrons involved in these ionic compounds.
Iron's Oxidation States and their Implications
The variable oxidation states of iron are directly related to its variable number of valence electrons. In the +2 oxidation state (ferrous), iron loses two electrons, typically from the 4s orbital. In the +3 oxidation state (ferric), iron loses three electrons, often involving one electron from the 4s orbital and two from the 3d orbital. This variation in oxidation states significantly influences iron's chemical reactivity and the properties of its compounds.
Ferrous (Fe²⁺) Compounds:
In ferrous compounds, iron loses two electrons, leaving it with a partially filled 3d orbital. This leads to specific magnetic and color properties often associated with ferrous compounds.
Ferric (Fe³⁺) Compounds:
In ferric compounds, iron loses three electrons, resulting in a more stable (half-filled) 3d orbital configuration. This configuration influences the color and other chemical properties of ferric compounds.
Importance of Iron's Valence Electrons
The variable number of valence electrons in iron is not simply an academic curiosity; it underpins many of its crucial properties and applications. This flexibility in bonding allows iron to form a wide range of compounds with diverse applications:
-
Steel Production: Iron's ability to readily bond with carbon and other alloying elements is fundamental to the production of steel, a material with immense structural and industrial importance. The number of valence electrons dictates the nature of the bonds formed, influencing the properties of the resulting steel alloy.
-
Biological Significance: Iron plays a vital role in biological systems. Hemoglobin, the oxygen-carrying protein in red blood cells, contains iron ions that facilitate oxygen transport. The variable oxidation states of iron within hemoglobin are essential for its function. The specific number of valence electrons influencing the interaction between iron and oxygen molecules.
-
Catalysis: Iron and its compounds are used extensively as catalysts in various chemical processes. The ability of iron to readily accept and donate electrons makes it an effective catalyst for numerous reactions, influencing reaction rates and selectivity. This catalytic activity is directly linked to its variable number of valence electrons.
-
Pigments: Iron oxides are widely used as pigments in paints, inks, and cosmetics. The different oxidation states of iron lead to different colors, with ferrous compounds often exhibiting greenish tints and ferric compounds exhibiting reddish or brownish colors. These colors are directly linked to the electronic transitions involving valence electrons.
Conclusion: A Deeper Understanding
Understanding the number of valence electrons in iron, and its variable nature, is crucial to grasping its diverse and essential roles in various fields. The complexity of transition metal chemistry, highlighted by iron's variable valence electron count, underscores the importance of considering the nuances of electronic configuration and orbital interactions when studying these elements. Further research and advancements in our understanding of iron's electronic properties continue to drive innovation and progress in materials science, biology, and many other disciplines. The seemingly simple question of how many valence electrons iron possesses opens up a wealth of complex and fascinating chemical and physical phenomena that continue to be investigated and appreciated.
Latest Posts
Latest Posts
-
What Is The Division Of The Cytoplasm Called
Mar 20, 2025
-
Is Air A Compound Or An Element
Mar 20, 2025
-
Is Rusting Iron A Chemical Or Physical Change
Mar 20, 2025
-
Reaction Of Ammonia With Sulphuric Acid
Mar 20, 2025
-
Least Common Multiple Of 4 And 12
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
