Number Of Valence Electrons In Chlorine Ion Are
Juapaving
Mar 23, 2025 · 5 min read
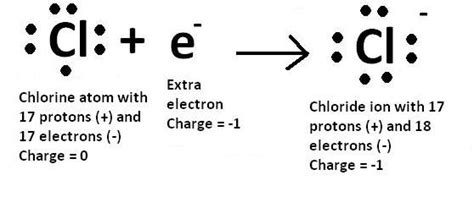
Table of Contents
The Number of Valence Electrons in a Chlorine Ion: A Deep Dive
Chlorine, a vibrant and reactive element, plays a crucial role in various chemical processes. Understanding its electronic structure, particularly the number of valence electrons in its ionic form, is fundamental to grasping its chemical behavior. This article delves into the intricacies of chlorine's valence electrons, explaining how it forms ions and the implications of its electronic configuration.
Understanding Valence Electrons
Before focusing on chlorine ions, let's establish a clear understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons dictates an atom's tendency to gain, lose, or share electrons to achieve a stable electron configuration, usually a full outer shell. This stable configuration is often referred to as the octet rule, where atoms strive to have eight electrons in their outermost shell (except for hydrogen and helium, which aim for two).
Chlorine's Electronic Structure
Chlorine (Cl), with an atomic number of 17, possesses 17 electrons. These electrons are arranged in specific energy levels or shells according to the Aufbau principle. The electronic configuration of a neutral chlorine atom is 1s²2s²2p⁶3s²3p⁵. This means:
- Shell 1 (n=1): Contains 2 electrons (1s²)
- Shell 2 (n=2): Contains 8 electrons (2s²2p⁶)
- Shell 3 (n=3): Contains 7 electrons (3s²3p⁵)
The outermost shell, shell 3, contains 7 valence electrons. This incomplete outermost shell makes chlorine highly reactive, as it strongly desires to achieve a stable octet.
Chlorine's Ion Formation: Achieving Stability
To attain a stable octet, chlorine readily gains one electron. This electron acquisition transforms the neutral chlorine atom into a chloride ion, denoted as Cl⁻. The process of gaining an electron is called reduction, as the chlorine atom reduces its overall positive charge.
The Chloride Ion (Cl⁻)
The addition of one electron to chlorine's outermost shell completes its octet. The electronic configuration of the chloride ion (Cl⁻) becomes 1s²2s²2p⁶3s²3p⁶. Notice how the third shell now holds a full complement of 8 electrons.
Crucially, the chloride ion (Cl⁻) has 8 valence electrons. The extra electron has filled the outermost shell, resulting in a stable, unreactive ion. This is a key aspect of chlorine's chemistry. The achievement of a stable octet is the driving force behind chlorine's reactivity and its tendency to form ionic compounds.
The Significance of Valence Electrons in Chlorine's Chemistry
The number of valence electrons in chlorine and its ion directly influences its chemical behavior:
-
Ionic Bonding: Chlorine's strong tendency to gain one electron enables it to form ionic bonds with metals. Metals, which tend to lose electrons, readily donate an electron to chlorine, resulting in the formation of a stable ionic compound. For example, the reaction between sodium (Na) and chlorine (Cl) forms sodium chloride (NaCl), common table salt. Sodium loses an electron to become Na⁺, and chlorine gains that electron to become Cl⁻. The electrostatic attraction between the oppositely charged ions forms the ionic bond.
-
Covalent Bonding: Although less common than ionic bonding, chlorine can also participate in covalent bonding. In covalent bonds, atoms share electrons to achieve a stable octet. Chlorine frequently forms single covalent bonds with other nonmetals, sharing one electron pair to achieve a full outer shell. For example, in hydrogen chloride (HCl), chlorine shares an electron pair with hydrogen.
-
Oxidation States: Chlorine's ability to gain an electron explains its common oxidation state of -1. Oxidation states represent the apparent charge of an atom in a compound. In ionic compounds containing chloride ions, the oxidation state of chlorine is -1, reflecting the single negative charge it carries. However, chlorine can exhibit other oxidation states in certain compounds, showcasing its versatility in bonding.
Comparing Neutral Chlorine and Chloride Ion
| Feature | Neutral Chlorine Atom (Cl) | Chloride Ion (Cl⁻) |
|---|---|---|
| Number of Electrons | 17 | 18 |
| Electronic Configuration | 1s²2s²2p⁶3s²3p⁵ | 1s²2s²2p⁶3s²3p⁶ |
| Number of Valence Electrons | 7 | 8 |
| Charge | 0 | -1 |
| Reactivity | High | Low |
| Stability | Unstable | Stable |
Applications and Importance
Chlorine and its compounds have extensive applications in various fields:
-
Water Purification: Chlorine is widely used to disinfect water, eliminating harmful bacteria and pathogens, ensuring safe drinking water for millions.
-
Industrial Processes: It plays a vital role in many industrial processes, including the production of plastics, solvents, and pharmaceuticals.
-
Medical Applications: Certain chlorine compounds have medicinal applications, contributing to the development of disinfectants and other medical products.
-
Household Products: Chlorine-based compounds are found in various household products, such as bleaches and cleaning agents.
Conclusion
The number of valence electrons in a chlorine ion (Cl⁻) is eight. This crucial detail explains chlorine's remarkable reactivity and its ability to form stable ionic and covalent compounds. The transition from a neutral chlorine atom with seven valence electrons to a chloride ion with a complete octet is fundamental to understanding chlorine's chemistry and its extensive applications in various fields. Understanding valence electrons is essential for comprehending chemical reactions and predicting the behavior of elements and compounds. The implications of chlorine's electronic structure extend far beyond the basic principles of chemistry, impacting our daily lives in numerous ways. From water purification to industrial processes, the impact of chlorine and its ions is undeniable.
Latest Posts
Latest Posts
-
What Are The Factors Of 200
Mar 25, 2025
-
5 Letter Words End With Er
Mar 25, 2025
-
How Many Cm Is 14 Inches
Mar 25, 2025
-
How Many Feet In A 100 Yards
Mar 25, 2025
-
Difference Between Nuclear Reaction And Chemical Reaction
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Chlorine Ion Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
