Melting Of Wax Is A Physical Or Chemical Change
Juapaving
Mar 31, 2025 · 5 min read
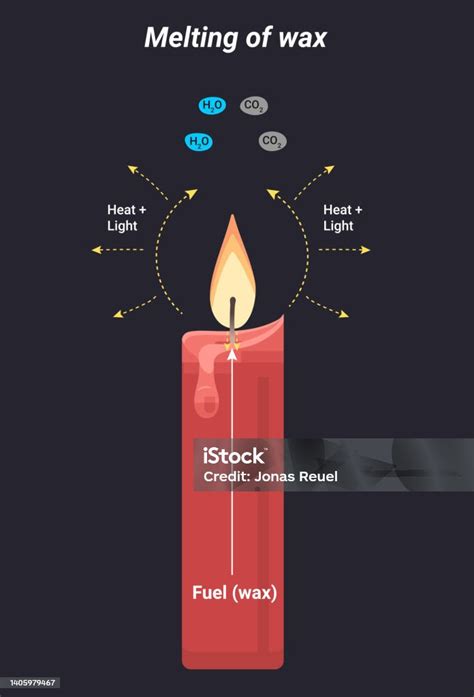
Table of Contents
Is Melting Wax a Physical or Chemical Change? A Comprehensive Exploration
The question of whether melting wax represents a physical or chemical change is a common one, often sparking debate among students and science enthusiasts alike. Understanding the difference between physical and chemical changes is crucial for grasping fundamental scientific concepts. This article delves deep into the process of wax melting, examining the evidence to definitively classify it as a physical change. We will explore the characteristics of both physical and chemical changes, analyze the properties of wax before and after melting, and debunk common misconceptions surrounding this seemingly simple phenomenon.
Understanding Physical and Chemical Changes
Before we tackle the specifics of wax, let's establish a clear understanding of the fundamental differences between physical and chemical changes.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same; only its physical properties, such as shape, size, or state of matter (solid, liquid, gas), are modified. These changes are often reversible. Examples include:
- Melting ice: Ice (solid water) melts into liquid water, but the chemical composition (H₂O) remains unchanged.
- Boiling water: Liquid water transforms into water vapor (gas), but it's still H₂O.
- Crushing a can: The can changes shape, but it's still made of the same metal.
Key characteristics of physical changes include:
- No new substance is formed.
- Changes are usually reversible.
- Only physical properties are altered (e.g., shape, size, state).
- No energy change (significant) is involved in most cases; the energy simply changes its form
Chemical Changes: A Transformation of Substance
A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into entirely new substances with different chemical properties. These changes are often irreversible and involve the breaking and forming of chemical bonds. Examples include:
- Burning wood: Wood reacts with oxygen to produce ash, smoke, and gases—completely different substances.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a new compound with different properties.
- Baking a cake: The ingredients undergo chemical reactions to form a new substance—the cake—with different properties than the individual ingredients.
Key characteristics of chemical changes include:
- New substances are formed.
- Changes are usually irreversible.
- Chemical properties are altered (e.g., reactivity, flammability).
- Energy is usually absorbed or released (e.g., heat, light).
Analyzing the Melting of Wax: A Physical Transformation
Now, let's apply this knowledge to the melting of wax. When wax is heated, it transitions from a solid to a liquid state. This change seems significant, but a closer examination reveals it to be a physical change.
Evidence Supporting a Physical Change
-
No New Substance is Formed: When wax melts, it doesn't transform into a chemically different substance. The chemical composition of the wax remains the same. Once cooled, the liquid wax solidifies back into its original form, demonstrating the reversibility of the process. This is a hallmark of a physical change.
-
Reversibility: The melting of wax is entirely reversible. Upon cooling, the liquid wax solidifies, returning to its original solid state. This contrasts sharply with chemical changes, which are often irreversible.
-
Only Physical Properties Change: The primary change during wax melting is its state of matter—from solid to liquid. Other physical properties, such as viscosity (resistance to flow), may also change, but the fundamental chemical structure remains intact. There's no change in the chemical bonds within the wax molecules.
-
Energy Change is Primarily Physical: Melting wax requires an input of heat energy to overcome the intermolecular forces holding the wax molecules together in a solid state. However, this energy change is primarily a physical process of changing the kinetic energy of the molecules rather than breaking or forming chemical bonds. The energy is released upon solidification.
Addressing Common Misconceptions
Some may argue that the color or scent of the wax might change slightly upon melting, suggesting a chemical alteration. However, these subtle changes are usually due to impurities in the wax or the release of volatile compounds and are not indicative of a fundamental chemical transformation of the wax itself. The core chemical structure remains unchanged. These are physical changes related to the state of the wax and its interactions with surrounding molecules.
Similarly, if the wax is burned (combustion), this is a chemical change, as the wax reacts with oxygen to produce carbon dioxide, water, and other byproducts. Burning is a completely different process than simply melting the wax.
Types of Wax and Their Melting Behavior
Different types of wax, such as paraffin wax, beeswax, soy wax, etc., may have slightly varying melting points and behaviors. However, the fundamental process remains the same: a physical change. The differences in melting points are due to variations in the molecular structure and chain length of the wax molecules, not a fundamental change in the type of chemical reaction involved.
The melting point itself is a physical property. The ability of a particular type of wax to melt at a specific temperature is related to the intermolecular forces present in its structure. A higher melting point indicates stronger intermolecular forces and conversely, lower melting points indicate weaker intermolecular forces.
Conclusion: Melting Wax is a Physical Phenomenon
In conclusion, the melting of wax is unequivocally a physical change. The process involves a change in the state of matter from solid to liquid without altering the chemical composition of the wax. The reversibility of the process, the lack of new substance formation, and the alteration of only physical properties firmly establish it as a physical transformation. While subtle changes in color or scent might occur due to impurities or volatile compounds, these don't negate the core physical nature of the melting process. Understanding this distinction is crucial for comprehending fundamental scientific principles and appreciating the subtle yet significant differences between physical and chemical changes in the world around us. Furthermore, the study of wax melting provides a valuable introductory example of phase transitions, a critical concept in physics and chemistry.
Latest Posts
Latest Posts
-
What Is The Greatest Common Factor Of 49
Apr 02, 2025
-
The First Organisms On Earth Were
Apr 02, 2025
-
Whats The Prime Factorization Of 12
Apr 02, 2025
-
The Property Of Letting Light Pass Through Something
Apr 02, 2025
-
What Are The Greatest Common Factors Of 75
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Melting Of Wax Is A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
