Measure Of The Quantity Of Matter
Juapaving
Apr 01, 2025 · 6 min read
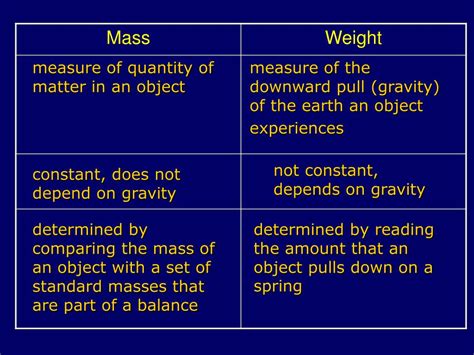
Table of Contents
Measuring the Quantity of Matter: A Deep Dive into Mass, Weight, and Moles
Understanding the quantity of matter is fundamental to chemistry and physics. While the terms "mass" and "weight" are often used interchangeably in everyday conversation, they represent distinct concepts crucial for accurate scientific measurement. This comprehensive guide delves into the intricacies of measuring the quantity of matter, exploring mass, weight, the mole concept, and their interrelationships.
What is Matter?
Before we delve into the methods of measuring matter, let's establish a clear understanding of what matter is. Matter is anything that occupies space and has mass. This encompasses everything around us – from the air we breathe to the ground beneath our feet, the stars in the sky, and even the smallest subatomic particles. Understanding the quantity of this matter is essential for various scientific endeavors.
Mass: The Inherent Property
Mass is a fundamental property of matter representing the amount of substance present in an object. It's a measure of an object's inertia – its resistance to changes in motion. A more massive object requires a greater force to accelerate it than a less massive object. Mass remains constant regardless of location – whether on Earth, the Moon, or in outer space. It's measured in kilograms (kg) in the International System of Units (SI). Other units include grams (g), milligrams (mg), and tonnes (t).
Measuring Mass: Common Tools
Several instruments accurately measure mass:
-
Analytical balances: These highly precise instruments are used in laboratories to measure mass with exceptional accuracy, often to several decimal places. They are crucial for precise chemical experiments and analyses.
-
Top-loading balances: These balances provide a good balance between accuracy and speed. They are commonly used in various settings, from laboratories to educational institutions.
-
Triple-beam balances: These mechanical balances utilize sliding weights to determine the mass of an object. Although less precise than electronic balances, they offer a valuable hands-on learning experience in basic mass measurement principles.
Weight: The Force of Gravity
Unlike mass, weight is a force resulting from the gravitational attraction between an object and a celestial body (like Earth). It's a vector quantity, possessing both magnitude and direction. Weight is calculated using the formula:
Weight = Mass × Acceleration due to gravity (g)
Where 'g' is approximately 9.8 m/s² on Earth. This means an object with a mass of 1 kg has a weight of approximately 9.8 Newtons (N) on Earth. However, on the Moon, where the gravitational acceleration is lower, the same object would weigh less despite having the same mass.
Measuring Weight: Scales and Force Gauges
Weight is commonly measured using:
-
Spring scales: These scales measure the force exerted by gravity on an object. The extension of a spring is proportional to the weight.
-
Electronic scales: These digital scales use strain gauges or load cells to precisely measure weight. They are widely used in various applications due to their accuracy and convenience.
-
Beam balances (equal-arm balance): Unlike spring scales, these balances compare the weight of an unknown object to known weights, providing a direct comparison rather than a measurement of force.
The Mole: A Chemist's Dozen
While mass provides a measure of the quantity of matter, chemists often need a way to quantify the number of atoms or molecules present. This is where the mole comes in. A mole (mol) is a fundamental unit in chemistry representing Avogadro's number (approximately 6.022 × 10²³) of particles. This number is the number of atoms in exactly 12 grams of carbon-12.
The mole allows chemists to relate macroscopic measurements (like mass) to microscopic quantities (like the number of atoms or molecules). The molar mass of a substance is the mass of one mole of that substance and is expressed in grams per mole (g/mol). For example, the molar mass of water (H₂O) is approximately 18 g/mol. This means that one mole of water contains approximately 6.022 × 10²³ water molecules and has a mass of 18 grams.
Calculating Moles: The Crucial Equation
The relationship between mass, molar mass, and the number of moles is expressed by the following equation:
Number of moles (n) = Mass (m) / Molar mass (M)
This equation is fundamental to many stoichiometric calculations in chemistry.
The Interplay of Mass, Weight, and Moles
These three concepts – mass, weight, and the mole – are interconnected, though distinct. Mass is an inherent property of matter, while weight depends on gravitational force. The mole provides a way to count the number of particles, linking the macroscopic world of mass measurements to the microscopic world of atoms and molecules.
For example, if we have 18 grams of water (m = 18 g) and we know the molar mass of water is 18 g/mol (M = 18 g/mol), we can calculate the number of moles using the equation above:
n = 18 g / 18 g/mol = 1 mol
This tells us that 18 grams of water contains one mole of water molecules, or approximately 6.022 × 10²³ water molecules. The weight of this water, however, would depend on the gravitational field it's in.
Advanced Concepts and Applications
The measurement of the quantity of matter extends beyond basic mass, weight, and mole calculations. More advanced concepts include:
-
Density: Density is the mass of a substance per unit volume. It is an important physical property used to identify substances and understand their properties.
-
Specific gravity: This is the ratio of the density of a substance to the density of a reference substance, typically water.
-
Volume: Determining the volume of a substance is crucial for calculating density and other related properties. Various methods exist for measuring volume, depending on the state of matter (solid, liquid, or gas).
-
Stoichiometry: This branch of chemistry deals with the quantitative relationships between reactants and products in chemical reactions. Molar masses and mole calculations are essential for stoichiometric calculations.
-
Isotopes and Atomic Mass: The concept of the mole becomes more complex when considering isotopes, which are atoms of the same element with different numbers of neutrons. The atomic mass used in molar mass calculations is a weighted average of the masses of all isotopes of an element.
Conclusion: Precision and Accuracy in Measurement
Accurate measurement of the quantity of matter is paramount in various scientific disciplines, particularly in chemistry and physics. Understanding the differences between mass and weight, and the importance of the mole concept, is essential for performing accurate calculations and interpreting experimental results. The appropriate tools and techniques must be selected based on the required precision and accuracy of the measurement. By mastering these concepts and employing the appropriate methodology, scientists can confidently quantify matter and unlock further understanding of the universe around us. Continual advancements in measuring instruments further enhance the precision and accuracy of these critical measurements, leading to a deeper understanding of the fundamental properties of matter.
Latest Posts
Latest Posts
-
Plant Is Where Photosynthesis Takes Place
Apr 02, 2025
-
12 Cm Is How Many Inches
Apr 02, 2025
-
The Most Abundant Gas In The Earths Atmosphere Is
Apr 02, 2025
-
Is Rubbing Alcohol And Denatured Alcohol The Same
Apr 02, 2025
-
Is 17 A Prime Number Or A Composite Number
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Measure Of The Quantity Of Matter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
