Law Of Conservation Of Mass Problems
Juapaving
Mar 27, 2025 · 6 min read
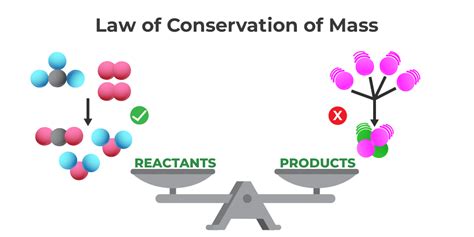
Table of Contents
Delving Deep into the Law of Conservation of Mass: Problems and Solutions
The Law of Conservation of Mass, a cornerstone of chemistry and physics, states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. This seemingly simple principle has profound implications across numerous scientific fields, and understanding its nuances is crucial for solving a wide array of problems. This article explores the law in detail, examining common problem types, offering solutions, and highlighting the importance of understanding its limitations.
Understanding the Fundamentals: What is Conservation of Mass?
Before diving into problem-solving, it's essential to fully grasp the law itself. The principle hinges on the idea of a closed system, meaning a system where no matter can enter or leave. In such a system, the total mass before a reaction or process will always equal the total mass after the reaction or process. This is true regardless of the chemical or physical changes that occur within the system.
Key Considerations for Conservation of Mass Problems:
- Closed System: This is paramount. Any leakage or addition of matter will violate the law within the context of that specific experiment or scenario.
- Accurate Measurements: Precise measurements of mass are crucial for verifying the law. Errors in measurement can lead to apparent violations.
- Unseen Products: Sometimes, products of a reaction might be in a different phase (e.g., a gas escaping) making it seem like mass is lost. Careful experimental design accounts for this.
- Nuclear Reactions: The Law of Conservation of Mass is strictly true only for chemical and physical processes that do not involve significant changes in nuclear structure. Nuclear reactions, which involve changes in the atomic nuclei, do not strictly obey the law because a small amount of mass is converted to energy (as described by Einstein's famous equation, E=mc²).
Common Types of Conservation of Mass Problems
Let's examine the various types of problems encountered when applying the Law of Conservation of Mass:
Type 1: Simple Mass Balance Problems
These problems often involve straightforward calculations where you are given the mass of reactants and asked to find the mass of products (or vice versa) in a closed system.
Example: A chemical reaction involves 10 grams of reactant A and 5 grams of reactant B. After the reaction, 12 grams of product C are formed. What is the mass of product D?
Solution: According to the law of conservation of mass, the total mass of reactants must equal the total mass of products. Therefore:
Mass of reactants = Mass of products
10g (A) + 5g (B) = 12g (C) + Mass of D
15g = 12g + Mass of D
Mass of D = 15g - 12g = 3g
Therefore, the mass of product D is 3 grams.
Type 2: Problems Involving Phase Changes
These problems often involve scenarios where a phase change (solid to liquid, liquid to gas, etc.) occurs. It’s crucial to remember that phase changes do not alter the total mass of the system.
Example: 20 grams of ice melts into liquid water. What is the mass of the liquid water?
Solution: The mass remains constant. The mass of the liquid water is also 20 grams.
Type 3: Problems with Unseen Products
These are more challenging as they require considering products that may not be readily apparent, such as gases produced in a reaction.
Example: A 5-gram sample of a carbonate mineral is heated, producing 2 grams of solid oxide and an unknown mass of carbon dioxide gas. What is the mass of the carbon dioxide?
Solution: The total mass of reactants must equal the total mass of products. Therefore:
Mass of reactant = Mass of solid oxide + Mass of carbon dioxide
5g = 2g + Mass of carbon dioxide
Mass of carbon dioxide = 5g - 2g = 3g
Therefore, 3 grams of carbon dioxide gas were produced.
Type 4: Problems Involving Multiple Reactions
These problems involve sequences of reactions where the product of one reaction becomes a reactant in the next. Careful tracking of mass throughout the entire process is essential.
Example: Reaction 1: A + B → C (10g A + 5g B produces 13g C) Reaction 2: C + D → E. If 2g of D is added to the 13g of C, and 14g of E is produced, what is the mass loss during reaction 2?
Solution:
- Reaction 1: Mass is conserved (10g + 5g = 13g)
- Reaction 2: Mass of reactants = 13g (C) + 2g (D) = 15g
- Mass of products = 14g (E)
- Mass loss = 15g - 14g = 1g
There's a 1g mass loss during reaction 2, possibly due to a gas or energy being released, highlighting the importance of a truly closed system for accurate mass conservation.
Type 5: Problems Addressing Limitations of the Law
These problems highlight the exceptions, primarily focusing on nuclear reactions.
Example: A nuclear reaction results in a decrease in mass. Explain why this does not violate the law of conservation of mass.
Solution: The decrease in mass is converted into energy, as described by Einstein's equation (E=mc²). While mass is not conserved in the strictest sense, the total energy-mass of the system remains constant. The law of conservation of mass-energy is a more accurate and comprehensive principle than just the law of conservation of mass when considering nuclear processes.
Advanced Problem-Solving Strategies
Addressing complex conservation of mass problems often requires employing several strategies:
- Visual Representation: Drawing diagrams or flowcharts to represent the process can help visualize mass changes.
- Step-by-Step Approach: Breaking down complex problems into smaller, more manageable steps simplifies the calculations.
- Careful Unit Conversion: Ensure all mass measurements are in the same units to prevent errors.
- Consider all products: Account for all substances (solids, liquids, gases) formed during the reaction or process.
- Understanding the Context: Clearly identify if the system is closed, open, or isolated, as this is crucial in the application of the law.
Real-World Applications of the Law of Conservation of Mass
The Law of Conservation of Mass isn't just a theoretical concept; it has wide-ranging practical applications:
- Stoichiometry: Calculations in chemical reactions rely heavily on this law to determine the amounts of reactants and products.
- Environmental Science: Tracking pollutants and assessing their impact on ecosystems relies on mass balance principles.
- Industrial Processes: Optimizing industrial processes requires careful consideration of mass balance to minimize waste and maximize efficiency.
- Forensic Science: Determining the origin or composition of materials often involves applying mass balance concepts.
Conclusion: The Enduring Importance of Conservation of Mass
The Law of Conservation of Mass, while seemingly straightforward, is a fundamental principle that underpins countless scientific advancements and technological applications. Understanding its nuances, along with the ability to solve various problem types, is critical for success in numerous scientific and engineering disciplines. By carefully considering the conditions of the system and employing effective problem-solving strategies, we can confidently apply this essential law to understand the world around us. Remember that while the law is a powerful tool, understanding its limitations, particularly when dealing with nuclear reactions, is crucial for a comprehensive grasp of mass and energy transformations in the universe. While this article provides a strong foundation, continued exploration and practical application will strengthen your understanding and problem-solving capabilities in this important area of science.
Latest Posts
Latest Posts
-
When Does Lhopitals Rule Not Apply
Mar 30, 2025
-
The Push Or Pull On An Object Is Called
Mar 30, 2025
-
Moment Of Inertia Of An Equilateral Triangle
Mar 30, 2025
-
Eat Is Regular Or Irregular Verb
Mar 30, 2025
-
The Principle Of Constant Proportions States That
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Law Of Conservation Of Mass Problems . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
