Isotopes Of An Element Differ In Their
Juapaving
Mar 15, 2025 · 7 min read
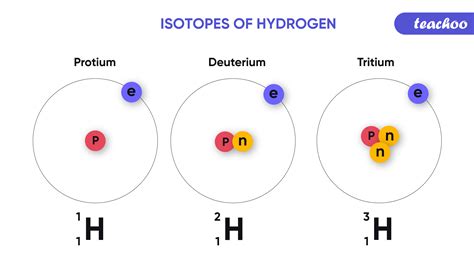
Table of Contents
Isotopes of an Element Differ in Their Neutron Number: A Deep Dive into Isotopic Variations
Isotopes are variations of a chemical element that possess the same number of protons but differ in the number of neutrons within their atomic nuclei. This seemingly subtle difference has profound implications for the element's properties, stability, and applications across various scientific fields. Understanding how isotopes differ is crucial to grasping nuclear chemistry, geochemistry, and even medical applications like radioisotope imaging. This article will delve into the intricacies of isotopic variation, exploring their impact on atomic mass, stability, and practical uses.
The Fundamental Difference: Neutron Count
The defining characteristic that distinguishes isotopes of the same element is the number of neutrons present in their nuclei. Recall that an atom's identity is determined by its atomic number, which represents the number of protons in the nucleus. All isotopes of a given element share the same atomic number. However, the mass number, which is the sum of protons and neutrons, varies between isotopes.
For example, consider carbon (C), which has an atomic number of 6. This means all carbon atoms possess 6 protons. However, carbon exists in nature as three primary isotopes:
- Carbon-12 (¹²C): Contains 6 protons and 6 neutrons (mass number = 12). This is the most abundant isotope of carbon.
- Carbon-13 (¹³C): Contains 6 protons and 7 neutrons (mass number = 13). A stable but less abundant isotope.
- Carbon-14 (¹⁴C): Contains 6 protons and 8 neutrons (mass number = 14). This is a radioactive isotope with a relatively short half-life.
This simple illustration highlights the fundamental difference: the neutron number. This seemingly small variation leads to significant differences in the properties and behavior of these isotopes.
Impact on Atomic Mass and Average Atomic Weight
The difference in neutron number directly affects the atomic mass of an isotope. Since neutrons contribute significantly to an atom's mass, isotopes with more neutrons have a higher atomic mass. This is why we use mass numbers (e.g., ¹²C, ¹³C, ¹⁴C) to distinguish isotopes.
The periodic table lists the average atomic weight for each element. This average is a weighted average of the atomic masses of all naturally occurring isotopes of that element, taking into account their relative abundances. For instance, the average atomic weight of carbon is approximately 12.011 amu (atomic mass units), reflecting the higher abundance of ¹²C compared to ¹³C and the trace amount of ¹⁴C.
Isotopic Stability and Radioactivity
The number of neutrons significantly influences an isotope's nuclear stability. Stable isotopes have a neutron-to-proton ratio that allows the nucleus to remain intact. However, many isotopes are radioactive, meaning their nuclei are unstable and undergo spontaneous decay to achieve a more stable configuration. This decay process involves emitting particles (like alpha, beta, or gamma radiation) or undergoing nuclear fission.
The stability of an isotope is related to the "strong nuclear force," which counteracts the electrostatic repulsion between protons. Too many or too few neutrons disrupt the balance of this force, leading to instability and radioactivity. This instability is particularly pronounced in heavier elements with high atomic numbers.
Radioactive Decay and Half-Life
Radioactive isotopes decay at a characteristic rate defined by their half-life. The half-life is the time it takes for half of the atoms in a sample to decay. Half-lives can range from fractions of a second to billions of years, depending on the isotope.
Radioactive decay is a random process at the individual atom level, but predictable for large ensembles of atoms. This predictability makes radioactive isotopes valuable tools in various applications, including:
- Radiometric dating: Determining the age of geological samples or artifacts by measuring the remaining amount of a radioactive isotope and its decay product. Carbon-14 dating is a prime example.
- Medical imaging and treatment: Radioactive isotopes are used in techniques like PET (positron emission tomography) scans and radiotherapy to diagnose and treat diseases.
- Industrial tracers: Radioactive isotopes can be used to track the movement of materials in industrial processes.
Isotopic Fractionation: Nature's Separation
Despite their similarities, isotopes do exhibit slight differences in their chemical and physical properties, a phenomenon known as isotopic fractionation. These differences are mainly due to the mass difference between isotopes. Heavier isotopes generally react slightly slower than lighter isotopes due to kinetic isotope effects.
Isotopic fractionation occurs naturally through various processes, including:
- Evaporation and condensation: Lighter isotopes tend to evaporate and condense more readily than heavier isotopes.
- Diffusion: Lighter isotopes diffuse faster than heavier isotopes.
- Biological processes: Some organisms preferentially utilize lighter isotopes over heavier ones during metabolism.
These fractionation processes lead to variations in the isotopic ratios of elements in different environments and organisms. The analysis of these variations provides valuable insights into various natural processes and historical events.
Applications of Isotopic Fractionation
The study of isotopic fractionation has numerous applications:
- Paleoclimatology: Analyzing the isotopic ratios of oxygen in ancient ice cores or sediments to reconstruct past climate conditions.
- Hydrology: Tracing the movement of water through different hydrological systems by analyzing the isotopic ratios of water molecules.
- Forensics: Analyzing the isotopic ratios of elements in materials to trace their origin or identify suspects.
- Food authenticity: Determining the geographic origin of food products based on the isotopic ratios of their constituents.
Isotope Effects in Chemical Reactions
Isotopic substitution can affect the rate and equilibrium of chemical reactions. This is because heavier isotopes have slightly different vibrational frequencies and bond strengths compared to their lighter counterparts. These differences can lead to kinetic isotope effects, where the reaction rates of isotopically substituted molecules differ.
For instance, in reactions involving bond breaking, the heavier isotope will react slower because it requires more energy to break the stronger bond. This can have implications in various chemical and biochemical processes.
Isotopes in Nuclear Medicine
Radioactive isotopes play a crucial role in nuclear medicine, providing tools for both diagnosis and treatment. Specific isotopes, depending on their decay characteristics and half-life, are carefully selected for particular applications.
Diagnostic applications: Radioactive tracers are used in medical imaging techniques like PET (positron emission tomography) and SPECT (single-photon emission computed tomography) to visualize internal organs and processes. These tracers emit radiation that can be detected by specialized scanners, revealing information about organ function, blood flow, and metabolic activity.
Therapeutic applications: Radioactive isotopes are also used in radiotherapy to target and destroy cancerous cells. These isotopes can be delivered to the tumor site through various methods, including targeted drug delivery or implantation of radioactive seeds. The radiation emitted by the isotope damages the cancerous cells, inhibiting their growth or killing them outright.
Isotopes in Other Scientific Fields
The applications of isotopes extend far beyond medicine. Their unique properties and behavior find applications in diverse fields:
- Geochronology: Radioactive decay of isotopes is essential for determining the age of rocks, minerals, and other geological materials.
- Environmental science: Isotopic tracers are used to study the movement of pollutants and the cycling of nutrients in ecosystems.
- Archaeology: Isotopic analysis helps determine the diet and lifestyle of ancient populations.
- Materials science: Isotopes are used to study the structure and properties of materials.
Conclusion: The Significance of Isotopic Variation
Isotopes, despite their seemingly minor difference in neutron count, have a profound impact on various aspects of chemistry, physics, geology, biology, and medicine. Understanding how isotopes differ is fundamental to advancing knowledge and developing applications in diverse fields. From radiometric dating to medical imaging, the unique properties of isotopes continue to provide invaluable tools for scientific discovery and technological advancement. The continued research and development in isotopic analysis promise further exciting breakthroughs in the years to come. Further exploration of specific isotopic systems and their applications would require dedicated research papers within their respective fields, however, this comprehensive overview provides a solid foundation for understanding the key differences and importance of isotopic variations.
Latest Posts
Latest Posts
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
-
Does Cold Air Go Up Or Down
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Of An Element Differ In Their . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
