Is Water An Element Or Compound
Juapaving
Mar 20, 2025 · 5 min read
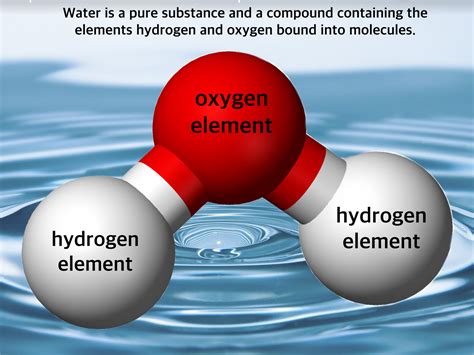
Table of Contents
Is Water an Element or a Compound? A Deep Dive into H₂O
The question, "Is water an element or a compound?" might seem simple at first glance. However, a deeper understanding requires exploring the fundamental concepts of elements, compounds, and the unique properties of water. This comprehensive article will delve into the chemical nature of water, clarifying its classification and highlighting its exceptional characteristics that stem from its molecular structure.
Understanding Elements and Compounds
Before we definitively classify water, let's establish clear definitions for elements and compounds.
What is an Element?
An element is a pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. This number of protons is known as the atomic number, and it uniquely identifies each element. Elements are the fundamental building blocks of all matter. They cannot be broken down into simpler substances by chemical means. Examples include oxygen (O), hydrogen (H), iron (Fe), and gold (Au). The periodic table organizes all known elements.
What is a Compound?
A compound, on the other hand, is a substance formed when two or more chemical elements are chemically bonded together. These bonds involve the sharing or transfer of electrons between atoms. The resulting compound has distinct properties from its constituent elements. Water, as we will see, is a prime example of a compound. Other common compounds include table salt (NaCl), carbon dioxide (CO₂), and sugar (C₁₂H₂₂O₁₁). Compounds can be broken down into simpler substances through chemical reactions.
The Chemical Composition of Water: Why Water is a Compound
Water, with its chemical formula H₂O, is composed of two elements: hydrogen and oxygen. Each molecule of water contains two hydrogen atoms covalently bonded to a single oxygen atom. This covalent bond involves the sharing of electrons between the hydrogen and oxygen atoms, resulting in a stable molecule.
Covalent Bonding in Water
The covalent bonds in water are crucial to understanding its properties. Oxygen is more electronegative than hydrogen, meaning it attracts electrons more strongly. This unequal sharing of electrons leads to a polar molecule, where one end (the oxygen side) carries a slightly negative charge (δ-), and the other end (the hydrogen side) carries a slightly positive charge (δ+). This polarity is responsible for many of water's unique characteristics, such as its high boiling point, surface tension, and ability to act as a solvent.
Water's Unique Properties: A Consequence of its Compound Nature
Water's properties are not simply the sum of the properties of hydrogen and oxygen. Its exceptional characteristics arise from its molecular structure and the interactions between water molecules.
-
High Boiling Point: Compared to other molecules of similar size, water has an unusually high boiling point (100°C at standard pressure). This is due to the strong hydrogen bonds between water molecules. Hydrogen bonds are a special type of intermolecular force that arises from the attraction between the slightly positive hydrogen atoms of one water molecule and the slightly negative oxygen atoms of another. These bonds require significant energy to break, leading to the high boiling point.
-
High Specific Heat Capacity: Water has a high specific heat capacity, meaning it can absorb a large amount of heat energy without a significant change in temperature. This property is crucial for regulating temperature in living organisms and in the Earth's climate. Again, hydrogen bonding plays a significant role, as it requires considerable energy to increase the kinetic energy of water molecules.
-
Excellent Solvent: Water is an excellent solvent for many ionic and polar substances. Its polarity allows it to interact with and dissolve charged particles, making it crucial for biological processes and chemical reactions. The polar nature of water molecules allows them to surround and effectively isolate ions, preventing them from recombining.
-
Surface Tension: Water exhibits high surface tension due to the strong cohesive forces between water molecules. These cohesive forces result from the hydrogen bonds, pulling water molecules together at the surface and creating a "skin-like" effect. This property is essential for the movement of water in plants and many other biological phenomena.
Debunking Misconceptions
Some might mistakenly consider water an element due to its ubiquitous nature and seeming simplicity. However, this is a misconception. The fact that water can be decomposed into its constituent elements (hydrogen and oxygen) through electrolysis (passing an electric current through water) is definitive proof that it is not an element. The process splits water into hydrogen gas and oxygen gas, showcasing its composite nature.
Water's Importance in Various Fields
The unique properties of water, stemming directly from its molecular structure as a compound, make it crucial across numerous fields:
-
Biology: Water is the solvent of life, acting as a medium for biological reactions and transporting nutrients and waste products in living organisms. Its high specific heat capacity helps regulate body temperature, while its cohesive and adhesive properties are vital for plant transport systems.
-
Chemistry: Water is a common reactant and solvent in countless chemical reactions. Its unique properties influence reaction rates and equilibrium. Many industrial processes rely heavily on water's solvent capabilities.
-
Environmental Science: The water cycle is fundamental to Earth's climate and ecosystems. Water's properties influence weather patterns, erosion, and the distribution of life on Earth. The study of water quality and pollution is a critical aspect of environmental protection.
-
Engineering: Water's properties are crucial in many engineering applications, including cooling systems, hydraulic systems, and construction. Understanding its behavior under different conditions is essential for designing effective and safe systems.
Conclusion: Water – A Vital Compound
In conclusion, water is unequivocally a compound, not an element. Its chemical formula, H₂O, clearly indicates its composition of two hydrogen atoms and one oxygen atom covalently bonded together. The unique properties of water – its high boiling point, specific heat capacity, solvent abilities, and surface tension – are direct consequences of this molecular structure and the resulting hydrogen bonding between molecules. This understanding is essential for appreciating water's crucial role in various scientific fields and its overall significance for life on Earth. The seemingly simple molecule of water is, in reality, a marvel of chemistry, a testament to the intricate workings of the natural world. Further research into its properties continues to unveil more of its complexities and importance. Therefore, it is crucial to understand that water, despite its simplicity, is a profoundly impactful compound with a multitude of crucial functions across various disciplines.
Latest Posts
Latest Posts
-
What Is 58 Inches In Feet
Mar 21, 2025
-
Least Common Multiple 3 And 4
Mar 21, 2025
-
How Much Is 25 Cm In Inches
Mar 21, 2025
-
What Kind Of Energy Transformation Happens In A Generator
Mar 21, 2025
-
How To Tell Wild Animals Mcq
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Is Water An Element Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
