Is Water A Mixture Or Compound
Juapaving
Mar 09, 2025 · 5 min read
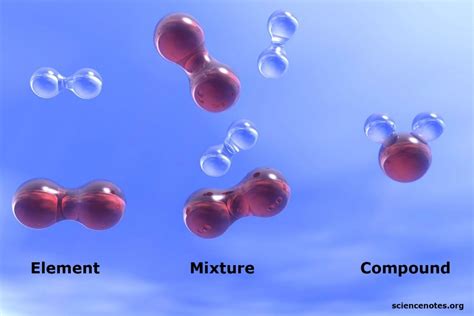
Table of Contents
Is Water a Mixture or a Compound? Understanding the Chemistry of H₂O
Water, the elixir of life, is a substance so ubiquitous that we often take its fundamental nature for granted. But the seemingly simple question, "Is water a mixture or a compound?" delves into the core concepts of chemistry and provides a fascinating exploration of molecular structure, bonding, and the properties of matter. The answer, while straightforward, opens the door to a deeper understanding of this essential molecule.
Defining Mixtures and Compounds
Before we dive into the classification of water, let's clearly define the key terms: mixture and compound.
Mixtures: A Blend of Substances
A mixture is a physical combination of two or more substances that are not chemically bonded. The individual components retain their original properties and can be separated by physical methods like filtration, distillation, or evaporation. Think of a salad: you have lettuce, tomatoes, cucumbers, and dressing—all mixed together, but each retaining its unique identity. Crucially, mixtures have variable compositions; the ratio of the components can change.
Examples of mixtures:
- Salt water: Salt dissolves in water, but the salt and water molecules are not chemically bound.
- Air: A mixture of various gases like nitrogen, oxygen, argon, and carbon dioxide.
- Sand and water: A heterogeneous mixture where sand particles are suspended in water.
Compounds: A Chemical Union
A compound, on the other hand, is a chemical combination of two or more elements that are chemically bonded in a fixed ratio. The properties of a compound are entirely different from the properties of its constituent elements. Breaking down a compound requires a chemical reaction, not a simple physical separation. Consider water (H₂O): its properties are vastly different from those of hydrogen and oxygen gases.
Examples of compounds:
- Water (H₂O): Two hydrogen atoms covalently bonded to one oxygen atom.
- Sodium chloride (NaCl): One sodium atom ionically bonded to one chlorine atom.
- Carbon dioxide (CO₂): One carbon atom covalently bonded to two oxygen atoms.
Water: A Definitive Compound
Given these definitions, the answer is clear: water is a compound. It's formed by the chemical combination of two hydrogen atoms and one oxygen atom, held together by strong covalent bonds. These bonds involve the sharing of electrons between the atoms, resulting in a stable molecule with distinct properties. You cannot simply separate hydrogen and oxygen from water through physical means; a chemical reaction is required, such as electrolysis.
The Covalent Bond in Water
The covalent bond in water is crucial to understanding its nature as a compound. Oxygen is highly electronegative, meaning it attracts electrons more strongly than hydrogen. This leads to a polar covalent bond, where the electrons are shared unequally, creating a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms.
This polarity is responsible for many of water's unique properties, including:
- High boiling point: The strong intermolecular forces (hydrogen bonds) between water molecules require significant energy to overcome, resulting in a relatively high boiling point.
- High surface tension: The cohesive forces between water molecules create a high surface tension, allowing insects to walk on water.
- Excellent solvent: The polarity of water allows it to dissolve many ionic and polar substances.
- High specific heat capacity: Water can absorb a significant amount of heat without a large temperature change, making it an excellent temperature regulator.
Understanding Hydrogen Bonding
Hydrogen bonding is a special type of intermolecular force that arises from the strong attraction between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another water molecule. These bonds are weaker than covalent bonds but strong enough to significantly influence water's properties.
Differentiating Water from Mixtures: A Closer Look
To further emphasize water's classification as a compound, let's compare it to mixtures that might seem similar at first glance:
- Saltwater: While saltwater appears homogeneous, it's a mixture. The salt (NaCl) is dissolved in water, but the ionic bonds within the salt crystals remain intact. Evaporation of saltwater leaves behind the salt, demonstrating the physical separation of the components.
- Muddy water: This is a heterogeneous mixture. The mud particles are suspended in water but not chemically bonded to it. Simple filtration can separate the mud from the water.
- Air: As mentioned earlier, air is a gaseous mixture. The components of air (nitrogen, oxygen, etc.) are not chemically bonded and can be separated through various physical processes like fractional distillation of liquid air.
In contrast to these mixtures, water's consistent chemical formula (H₂O) and the strong covalent bonds within its molecules definitively classify it as a compound. Its properties are unique and not simply an average of the properties of hydrogen and oxygen.
The Importance of Water's Compound Nature
The fact that water is a compound, not a mixture, has profound implications for life on Earth. Its unique properties, stemming directly from its molecular structure and bonding, are essential for biological processes:
- Solvent for biological reactions: Water's solvent properties allow for the transport of nutrients, the regulation of temperature, and the facilitation of countless biochemical reactions within living organisms.
- Medium for cellular processes: Water is the primary medium for cellular processes, enabling the movement of molecules and ions within cells.
- Participant in vital reactions: Water itself participates in many essential biological reactions, such as photosynthesis and respiration.
Conclusion: Water – A Compound of Vital Importance
The question of whether water is a mixture or a compound is a fundamental one that highlights the crucial distinction between physical and chemical combinations of matter. The unequivocal answer is that water is a compound. Its consistent chemical formula (H₂O), the strong covalent bonds holding its atoms together, and its unique properties, all stemming from this molecular structure, solidify its classification as a compound. Furthermore, understanding this fundamental chemical nature is crucial to appreciating water's unparalleled importance as the foundation of life on Earth. Its properties, directly linked to its compound nature, are essential for numerous biological processes and make it indispensable to all known life forms.
Latest Posts
Latest Posts
-
How Many Liters Is 3 Gallons
Mar 10, 2025
-
How Do You Calculate The Perimeter Of A Triangle
Mar 10, 2025
-
Which Of The Following Statements Is Incorrect
Mar 10, 2025
-
In What Organelle Does Cellular Respiration Occur
Mar 10, 2025
-
What Is The Squar Root Of 49
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Mixture Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
