Is Water A Compound Element Or Mixture
Juapaving
Mar 05, 2025 · 6 min read
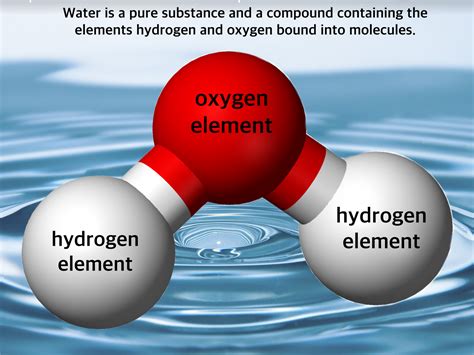
Table of Contents
Is Water a Compound, an Element, or a Mixture? A Deep Dive into the Nature of H₂O
The question of whether water is a compound, an element, or a mixture is a fundamental one in chemistry. Understanding the difference between these classifications is crucial to grasping the basic building blocks of matter. While the answer might seem simple at first glance, a closer examination reveals a deeper understanding of chemical bonding, molecular structure, and the properties of substances. This article will delve into the intricacies of water's nature, explaining why it's definitively a compound, not an element or a mixture.
Understanding the Basic Classifications of Matter
Before diving into the specifics of water, let's define the three classifications: element, compound, and mixture.
Element: The Fundamental Building Blocks
An element is a substance that cannot be broken down into simpler substances by chemical means. Elements are composed of only one type of atom. Examples include oxygen (O), hydrogen (H), iron (Fe), and gold (Au). The periodic table organizes all known elements. Each element has a unique atomic number, representing the number of protons in its nucleus.
Compound: A Union of Elements
A compound is a substance formed when two or more elements chemically combine in a fixed ratio. This combination involves the formation of chemical bonds, where atoms share or transfer electrons. The properties of a compound are distinctly different from the properties of its constituent elements. For instance, sodium (a highly reactive metal) and chlorine (a toxic gas) combine to form sodium chloride (table salt), a harmless crystalline solid. The chemical formula of a compound represents the ratio of elements in its structure; for example, H₂O for water.
Mixture: A Physical Blend, Not a Chemical Reaction
A mixture is a combination of two or more substances that are physically mixed but not chemically combined. The components of a mixture retain their individual properties and can be separated by physical methods like filtration, distillation, or evaporation. Examples include saltwater (a mixture of salt and water), air (a mixture of gases), and soil (a mixture of minerals and organic matter).
Why Water is a Compound, Not an Element or a Mixture
Water (H₂O) is unequivocally a compound because it meets the criteria of a compound's definition.
Evidence Supporting Water as a Compound:
-
Fixed Ratio of Elements: Water always contains hydrogen and oxygen in a precise 2:1 ratio. This ratio is consistent regardless of the source of the water – whether it's from a river, ocean, or even created in a laboratory. This fixed ratio is a hallmark of chemical compounds.
-
Distinct Properties: The properties of water (e.g., its liquid state at room temperature, its high boiling point, its ability to act as a solvent) are significantly different from the properties of its constituent elements, hydrogen (a highly flammable gas) and oxygen (a gas essential for respiration). This difference in properties demonstrates a chemical change has occurred.
-
Chemical Bonds: Water molecules are held together by strong covalent bonds. These bonds involve the sharing of electrons between hydrogen and oxygen atoms. This sharing of electrons is a defining characteristic of chemical compounds. It's a chemical interaction, not merely a physical association.
-
Decomposition Requires Chemical Processes: Water cannot be separated into hydrogen and oxygen by simple physical methods like boiling or filtering. Instead, it requires an electrochemical process, such as electrolysis, which utilizes an electric current to break the covalent bonds. This necessity for a chemical process further underscores water's status as a compound.
Why Water Isn't an Element:
Water is not an element because it can be broken down into simpler substances (hydrogen and oxygen) through chemical means. Remember, elements are substances that cannot be broken down chemically into simpler substances.
Why Water Isn't a Mixture:
Water is not a mixture because its components, hydrogen and oxygen, are chemically bonded. In a mixture, the components are physically mixed but retain their individual identities and properties. Water's unique properties are a direct result of the chemical bond between hydrogen and oxygen atoms. You cannot simply separate water into hydrogen and oxygen by physical means.
The Chemical Structure of Water: Understanding the Covalent Bond
The chemical structure of water plays a crucial role in understanding why it's a compound. Each water molecule consists of two hydrogen atoms covalently bonded to a single oxygen atom. The oxygen atom is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This leads to a polar covalent bond, where the oxygen atom carries a slightly negative charge (δ-), and the hydrogen atoms carry slightly positive charges (δ+). This polarity is responsible for many of water's unique properties, such as its high boiling point, surface tension, and its ability to act as a universal solvent.
The slightly positive hydrogen atoms of one water molecule are attracted to the slightly negative oxygen atoms of neighboring water molecules. This attraction, known as hydrogen bonding, is a relatively weak intermolecular force, but it significantly impacts water's properties, making it a liquid at room temperature rather than a gas like many other molecules of similar size.
The Significance of Water as a Compound
The fact that water is a compound is of profound significance in many aspects of life and the natural world.
-
Biological Importance: Water is essential for life. Its unique properties, stemming from its compound nature, make it an excellent solvent, facilitating biochemical reactions within cells. It plays vital roles in transporting nutrients, regulating temperature, and maintaining the structure of biological molecules.
-
Environmental Impact: Water's properties influence weather patterns, climate regulation, and the erosion and weathering of rocks. The unique behavior of water as a compound shapes the landscapes we inhabit.
-
Industrial Applications: Water's properties as a solvent are used extensively in various industries, from cleaning and manufacturing processes to power generation and chemical reactions.
Addressing Common Misconceptions
Some might confuse water with solutions, such as saltwater. While saltwater is a solution (a homogeneous mixture of salt and water), the water itself within that mixture remains a compound. The salt dissolves in the water, but it doesn't chemically react with the water to form a new compound. The water molecules remain intact as H₂O.
Another misconception involves isotopes. Water can exist with different isotopes of hydrogen and oxygen (e.g., deuterium oxide or "heavy water"). However, these variations do not change the fundamental nature of water as a compound. The isotopes differ in the number of neutrons in their nuclei, but the chemical bonding and molecular structure remain the same.
Conclusion: Water - A Fundamental Compound of Life
In conclusion, the evidence overwhelmingly supports the classification of water as a compound. Its fixed ratio of hydrogen and oxygen, its distinct properties, its covalent bonding, and the need for chemical processes to decompose it all point towards this classification. Understanding the nature of water as a compound is crucial for appreciating its unique properties and its fundamental role in our world and in the existence of life as we know it. The seemingly simple question of whether water is a compound, element, or mixture leads to a deeper exploration of chemical bonding, molecular structure, and the profound impact of a simple molecule on the universe.
Latest Posts
Latest Posts
-
How Many Feet Is 40 Inches
Mar 05, 2025
-
Alternate Forms Of The Same Gene Are Called
Mar 05, 2025
-
Radius Of Convergence And Interval Of Convergence Calculator
Mar 05, 2025
-
60 Inches Is How Many Feet
Mar 05, 2025
-
Is Rusting Iron A Chemical Change
Mar 05, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Compound Element Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
