Is The Final Electron Acceptor Of The Electron Transport Chain
Juapaving
Mar 07, 2025 · 6 min read
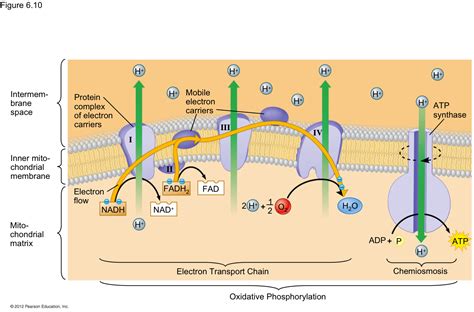
Table of Contents
Is Oxygen the Final Electron Acceptor of the Electron Transport Chain? A Deep Dive into Cellular Respiration
The electron transport chain (ETC), a crucial component of cellular respiration, is a complex series of protein complexes embedded within the inner mitochondrial membrane. Its primary function is to harness the energy stored in electrons derived from the breakdown of glucose and other fuel molecules to generate a proton gradient, which ultimately drives ATP synthesis – the energy currency of the cell. While the question of the final electron acceptor is often simplified to "oxygen," a closer examination reveals a more nuanced and fascinating story.
Understanding the Electron Transport Chain: A Step-by-Step Approach
Before we delve into the final electron acceptor, let's review the fundamental workings of the ETC. The process begins with the transfer of high-energy electrons from NADH and FADH₂, molecules produced during glycolysis and the citric acid cycle (Krebs cycle). These electron carriers donate their electrons to a series of protein complexes (Complex I-IV) located within the inner mitochondrial membrane.
Complex I: NADH Dehydrogenase
Complex I, also known as NADH dehydrogenase, accepts electrons from NADH. This electron transfer initiates a series of redox reactions, transferring electrons down the chain and simultaneously pumping protons (H⁺) from the mitochondrial matrix across the inner mitochondrial membrane into the intermembrane space. This creates an electrochemical gradient, storing potential energy.
Complex II: Succinate Dehydrogenase
Complex II, succinate dehydrogenase, is slightly different. Unlike Complex I, it doesn't pump protons directly. It receives electrons from FADH₂ (produced during the citric acid cycle) and passes them along the chain to Complex III.
Complex III: Cytochrome bc₁ Complex
Complex III, the cytochrome bc₁ complex, accepts electrons from Complex I or Complex II. This complex also participates in proton pumping, further contributing to the electrochemical gradient. The movement of electrons through Complex III is crucial for the establishment of the proton gradient.
Complex IV: Cytochrome c Oxidase
Complex IV, cytochrome c oxidase, is the final protein complex in the ETC. It receives electrons from cytochrome c, a mobile electron carrier that shuttles electrons between Complex III and Complex IV. This complex plays a pivotal role in the reduction of molecular oxygen (O₂), the quintessential final electron acceptor in aerobic respiration.
The Role of Oxygen: The Terminal Electron Acceptor in Aerobic Respiration
Oxygen's role as the terminal electron acceptor is paramount. Without it, the electron transport chain grinds to a halt. The electrons, having traversed the chain and lost their energy, are finally passed to oxygen, which is reduced to water (H₂O). This process is crucial for several reasons:
-
Maintaining the Electron Flow: The continuous flow of electrons through the ETC depends on the availability of a final acceptor. Oxygen's high electronegativity makes it an ideal terminal acceptor, ensuring a steady flow of electrons. Without oxygen to accept these electrons, the entire chain becomes backed up, and ATP production ceases.
-
Preventing Oxidative Stress: If the ETC were to halt without a final electron acceptor, electrons could leak out and react with oxygen, forming highly reactive oxygen species (ROS). These ROS are dangerous, damaging cellular components and contributing to oxidative stress and cell death.
-
Regenerating NAD⁺ and FAD: The ETC is inextricably linked to the citric acid cycle and glycolysis. The regeneration of NAD⁺ and FAD, the oxidized forms of the electron carriers, is dependent on the ETC's ability to accept electrons. Without oxygen, these molecules remain reduced, halting the cycle and limiting energy production.
Alternative Electron Acceptors: Anaerobic Respiration
While oxygen is the most common and efficient final electron acceptor, some organisms can utilize alternative electron acceptors under anaerobic conditions (without oxygen). This process is known as anaerobic respiration, and it yields significantly less ATP than aerobic respiration.
-
Nitrate (NO₃⁻): Certain bacteria can use nitrate as a final electron acceptor. Nitrate reductase catalyzes the reduction of nitrate to nitrite (NO₂⁻) or other nitrogenous compounds. This process is vital in the nitrogen cycle.
-
Sulfate (SO₄²⁻): Sulfate-reducing bacteria can use sulfate as an electron acceptor, reducing it to hydrogen sulfide (H₂S). This process is significant in sulfur cycling and can contribute to the formation of certain minerals.
-
Carbon Dioxide (CO₂): Some archaea utilize carbon dioxide as the final electron acceptor in a process called methanogenesis, producing methane (CH₄) as a byproduct. Methanogens play a crucial role in anaerobic environments like swamps and the digestive tracts of ruminants.
-
Fumarate: Certain bacteria and archaea can employ fumarate as an electron acceptor, reducing it to succinate.
The Importance of the Final Electron Acceptor: A Systemic View
The final electron acceptor's significance extends far beyond the ETC itself. It is a crucial component of the larger ecosystem of cellular respiration and energy production. The entire process is intricately linked, with the availability of the final electron acceptor dictating the efficiency and capacity of the entire system. Understanding the role of the final electron acceptor is fundamental to comprehending the complexities of cellular metabolism and energy generation in living organisms.
Implications of an Absent or Inefficient Final Electron Acceptor
The absence of an efficient final electron acceptor, particularly oxygen in aerobic organisms, leads to severe consequences. The most immediate effect is the cessation of ATP production through oxidative phosphorylation. This energy deficit can lead to cell death. Furthermore, the buildup of reduced electron carriers (NADH and FADH₂) prevents the continuation of glycolysis and the citric acid cycle, further hindering energy production.
Beyond the Basics: Exploring Further Research
The electron transport chain remains a subject of ongoing research. Scientists are constantly seeking a deeper understanding of the intricate mechanisms involved in electron transfer, proton pumping, and the regulation of the ETC. Research into the structure and function of the protein complexes, the role of various cofactors, and the impacts of mutations and inhibitors is crucial for developing treatments for various diseases and for understanding fundamental biological processes.
Conclusion: Oxygen, the Efficient and Essential Final Electron Acceptor
While alternative electron acceptors exist, oxygen remains the most efficient and prevalent final electron acceptor in the vast majority of living organisms. Its high electronegativity ensures a continuous flow of electrons, maximizing ATP production and preventing the harmful effects of oxidative stress. Understanding the role of oxygen, and the alternative acceptors in anaerobic respiration, is crucial to understanding the fundamental principles of cellular respiration and the diversity of metabolic strategies found in the living world. The intricate dance of electrons through the ETC, culminating in the reduction of the final electron acceptor, highlights the remarkable efficiency and elegance of biological systems. The entire process underscores the interconnectedness of cellular processes and the vital role of the final electron acceptor in supporting life as we know it.
Latest Posts
Latest Posts
-
Is 81 A Prime Or Composite Number
Mar 09, 2025
-
When Diluting An Acid With Water
Mar 09, 2025
-
What Is The Opposite Of Heavy
Mar 09, 2025
-
In Which Of The Following Organelles Does Photosynthesis Take Place
Mar 09, 2025
-
How To Find Base Of A Parallelogram
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about Is The Final Electron Acceptor Of The Electron Transport Chain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
