Two Or More Elements Chemically Combined
Juapaving
Mar 10, 2025 · 6 min read
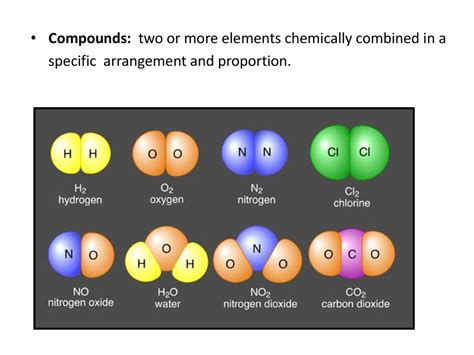
Table of Contents
Two or More Elements Chemically Combined: A Deep Dive into Compounds
When two or more elements chemically combine, they form a compound. This seemingly simple statement belies a world of complexity and fascinating properties. Unlike mixtures, where elements retain their individual identities, compounds represent a fundamental transformation – a new substance with unique characteristics distinct from its constituent elements. This article delves deep into the nature of compounds, exploring their formation, properties, types, and significance in various fields.
Understanding Chemical Bonds: The Glue that Holds Compounds Together
The very essence of a compound lies in the chemical bonds that unite its constituent elements. These bonds arise from the electrostatic interactions between atoms, driven by the desire to achieve a stable electron configuration, often resembling that of a noble gas. There are several primary types of chemical bonds:
1. Ionic Bonds: The Electrostatic Attraction
Ionic bonds form when one atom transfers one or more electrons to another atom. This transfer creates ions: positively charged cations (electron donors) and negatively charged anions (electron acceptors). The strong electrostatic attraction between these oppositely charged ions constitutes the ionic bond. Ionic compounds, often crystalline solids at room temperature, are typically formed between metals (which readily lose electrons) and nonmetals (which readily gain electrons). Examples include sodium chloride (NaCl, common table salt) and magnesium oxide (MgO).
Key Characteristics of Ionic Compounds:
- High melting and boiling points: Due to the strong electrostatic forces between ions.
- Brittle: Stress can cause misalignment of ions, leading to repulsion and fracture.
- Conduct electricity when molten or dissolved: Free-moving ions can carry an electric current.
- Often soluble in polar solvents: Like dissolves like – polar solvents can interact with the charged ions.
2. Covalent Bonds: Sharing is Caring
Covalent bonds involve the sharing of electrons between atoms. This sharing allows both atoms to achieve a stable electron configuration. Covalent compounds are typically formed between nonmetals. Examples include water (H₂O), methane (CH₄), and carbon dioxide (CO₂).
Key Characteristics of Covalent Compounds:
- Variable melting and boiling points: Ranging from gases (e.g., CO₂) to liquids (e.g., H₂O) to solids (e.g., sugar). Melting and boiling points depend on the strength of intermolecular forces.
- Generally poor conductors of electricity: Electrons are localized in shared bonds, not free to move.
- Solubility varies: Some are soluble in polar solvents, others in nonpolar solvents.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals. In this type of bonding, valence electrons are delocalized, forming a "sea" of electrons that surrounds the positively charged metal ions. This "sea" of electrons allows metals to exhibit unique properties.
Key Characteristics of Metallic Compounds:
- High electrical conductivity: The delocalized electrons are free to move and carry current.
- High thermal conductivity: The delocalized electrons efficiently transfer heat.
- Malleable and ductile: The "sea" of electrons allows atoms to slide past each other without disrupting the bond.
- Lustrous: The delocalized electrons interact with light, giving metals their characteristic shine.
Types of Compounds: A Diverse Chemical Landscape
Compounds are incredibly diverse, encompassing a vast array of chemical structures and properties. Several key classifications help categorize this vast landscape:
1. Organic Compounds: The Carbon Backbone
Organic compounds contain carbon atoms bonded to other carbon atoms or to hydrogen atoms. They form the basis of life and are found in countless naturally occurring and synthetic substances. Organic chemistry is a vast field dedicated to the study of these compounds. Examples include carbohydrates, lipids, proteins, and nucleic acids.
Key Characteristics of Organic Compounds:
- Covalent bonding: Predominantly covalent bonds between carbon and other atoms.
- Isomerism: The existence of molecules with the same chemical formula but different arrangements of atoms.
- Functional groups: Specific groups of atoms that confer characteristic properties to organic molecules.
2. Inorganic Compounds: The Rest of the World
Inorganic compounds encompass all compounds that are not organic. They typically lack a carbon-hydrogen framework and are found in minerals, salts, and numerous other materials. Examples include sodium chloride (NaCl), water (H₂O), and silicon dioxide (SiO₂, quartz).
3. Binary Compounds: Simplicity in Pairs
Binary compounds are composed of only two elements. They can be ionic (e.g., NaCl) or covalent (e.g., H₂O).
4. Ternary Compounds and Beyond: Increasing Complexity
Ternary compounds contain three elements, and the complexity continues to increase with the number of elements.
Naming Compounds: A Systematic Approach
The naming of compounds follows a systematic approach based on the type of bonding and the elements involved. This ensures clarity and consistency across the scientific community. For ionic compounds, the cation is named first, followed by the anion. For covalent compounds, prefixes (mono-, di-, tri-, etc.) are used to indicate the number of atoms of each element.
The Significance of Compounds: Shaping Our World
Compounds are fundamental to life, technology, and the environment. Their unique properties underpin countless applications:
- Medicine: Pharmaceuticals are largely composed of organic and inorganic compounds with specific biological activities.
- Materials science: The development of new materials with tailored properties relies on understanding and manipulating the properties of compounds. This includes polymers, ceramics, and composites.
- Agriculture: Fertilizers and pesticides are compounds designed to enhance crop yields and protect plants from pests.
- Energy: Fuels, such as hydrocarbons, are organic compounds that provide energy. Batteries and fuel cells also rely on electrochemical reactions involving various compounds.
- Environmental science: Understanding the chemical composition of air, water, and soil is crucial for environmental monitoring and remediation.
Chemical Reactions and Compound Formation
Compounds are formed through chemical reactions, processes that involve the rearrangement of atoms and the breaking and formation of chemical bonds. Chemical equations represent these reactions, showing the reactants (starting materials) and products (resulting compounds). Stoichiometry, the study of quantitative relationships in chemical reactions, allows us to predict the amounts of reactants and products involved.
Advanced Concepts: Exploring the Depths of Chemical Bonding
The concepts of chemical bonding extend beyond the basic models. Resonance structures, hybridization, and molecular orbital theory provide a more sophisticated understanding of how electrons are distributed in molecules and how this affects their properties. These advanced concepts are crucial for understanding the behavior of complex compounds and predicting their reactivity.
The Future of Compound Discovery and Application
The ongoing exploration of chemical space – the vast number of possible compounds – continues to reveal new molecules with remarkable properties. Computational chemistry and high-throughput screening techniques are accelerating the discovery of novel compounds with potential applications in medicine, materials science, and other fields. This ongoing research promises to revolutionize numerous aspects of our lives.
Conclusion: A World Built on Compounds
From the air we breathe to the food we eat, from the clothes we wear to the technology we use, compounds are integral to every aspect of our existence. Understanding the principles of chemical bonding and the properties of compounds is essential for advancing our knowledge and developing innovative solutions to the challenges facing humanity. The continuous exploration of the diverse world of compounds will continue to unlock new possibilities and shape the future.
Latest Posts
Latest Posts
-
Does The Diagonals Of A Parallelogram Bisect Each Other
May 09, 2025
-
Is 88 A Prime Or Composite Number
May 09, 2025
-
Which Of The Following Bonds Is The Weakest
May 09, 2025
-
Least Common Multiple Of 36 And 54
May 09, 2025
-
Compare And Contrast Weathering And Erosion
May 09, 2025
Related Post
Thank you for visiting our website which covers about Two Or More Elements Chemically Combined . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.