Is Starch A Polymer Of Glucose
Juapaving
Mar 19, 2025 · 6 min read
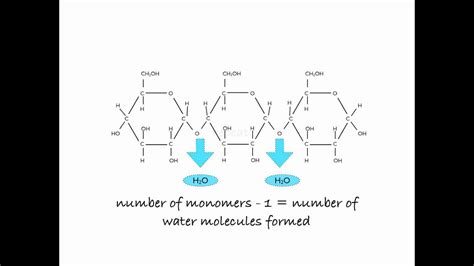
Table of Contents
Is Starch a Polymer of Glucose? A Deep Dive into Starch Structure and Function
Starch, a ubiquitous carbohydrate in our diet and a crucial energy storage molecule in plants, is indeed a polymer of glucose. However, understanding this seemingly simple statement requires a deeper exploration into the complexities of starch's structure, its various forms, and its biological significance. This article will delve into the chemical makeup of starch, detailing its polymeric nature and exploring the different types of glucose linkages that define its properties. We'll also investigate its role in plant biology and its implications for human nutrition and industrial applications.
Understanding Polymers and Glucose
Before delving into the specifics of starch, let's establish a foundation. A polymer is a large molecule composed of repeating smaller units called monomers. Think of it like a long chain made of many identical or similar links. In the case of starch, the monomer is glucose, a simple sugar with the chemical formula C₆H₁₂O₆. Glucose exists in several forms, most notably its linear (open-chain) and cyclic (ring) structures. It's the cyclic form, specifically the α-D-glucose, that serves as the building block for starch.
The Significance of α-D-Glucose
The specific configuration of glucose is crucial. The α and β forms of glucose differ in the orientation of the hydroxyl (-OH) group on carbon atom 1 (the anomeric carbon). This seemingly small difference has profound consequences for the resulting polymer's properties. Starch is composed entirely of α-D-glucose units, while cellulose, another crucial glucose polymer, utilizes β-D-glucose. This difference in linkage dictates the vastly different properties and functions of these two important polysaccharides.
Starch: A Mixture of Amylose and Amylopectin
Starch isn't a single homogeneous polymer but rather a mixture of two distinct glucose polymers: amylose and amylopectin. The proportion of each varies depending on the plant source.
Amylose: The Linear Chain
Amylose is a relatively linear polymer of α-D-glucose units linked together by α-1,4-glycosidic bonds. This means that the carbon atom 1 of one glucose molecule is linked to the carbon atom 4 of the next glucose molecule through an oxygen atom. These bonds create a long, unbranched chain, although the chain isn't perfectly straight; it adopts a helical conformation due to the angles of the glycosidic bonds. Amylose typically accounts for 20-30% of starch in most plants.
Amylopectin: The Branched Structure
Amylopectin, unlike amylose, is highly branched. While it also contains predominantly α-1,4-glycosidic bonds linking the glucose units, it also incorporates α-1,6-glycosidic bonds at branch points. These branch points occur approximately every 24-30 glucose units along the chain. This branching creates a complex, three-dimensional structure, significantly increasing the polymer's overall size and influencing its properties. Amylopectin constitutes the major portion of starch, usually 70-80%.
The Properties of Starch: A Consequence of Structure
The different structures of amylose and amylopectin directly influence the properties of starch. These properties are crucial for its function as an energy storage molecule and also determine its various industrial applications.
Solubility and Digestibility
Amylose, with its relatively linear structure, is less soluble in water than amylopectin. However, both amylose and amylopectin are readily digestible by humans and animals. The enzymes in our digestive systems, specifically amylases, break down the α-1,4-glycosidic bonds, releasing individual glucose molecules that can then be absorbed and used for energy. The branched structure of amylopectin allows for more simultaneous points of enzymatic attack, leading to faster digestion compared to amylose.
Gelatinization and Retrogradation
When starch is heated in water, it undergoes gelatinization. The ordered structure of starch granules breaks down, and water molecules penetrate the structure, causing swelling and increased viscosity. This process is crucial in food preparation, as it contributes to the thickening of sauces, gravies, and other food products.
After gelatinization, if the starch-water mixture is cooled, retrogradation can occur. This process involves the re-ordering of amylose and amylopectin molecules, leading to the expulsion of water and an increase in firmness or gelling. Retrogradation is responsible for the staling of bread and the syneresis (separation of liquid) in certain gels.
Starch Granule Structure: A Closer Look
Starch is not simply a random collection of amylose and amylopectin molecules. It's organized into starch granules, which are semi-crystalline structures with a complex internal organization. The arrangement of amylose and amylopectin within these granules significantly impacts starch properties, including its gelatinization and retrogradation behavior. The size and shape of starch granules vary depending on the plant source, influencing the texture and functionality of the starch in food applications.
The Biological Role of Starch
Starch serves a vital role as the primary energy storage polysaccharide in plants. It's synthesized in leaves during photosynthesis and then transported to various plant parts, such as roots, stems, and seeds, where it is stored in the form of starch granules. When the plant needs energy, starch is broken down into glucose through enzymatic hydrolysis, providing the necessary fuel for metabolic processes. This energy storage is crucial for plant growth, development, and survival.
Starch in Human Nutrition and Industry
Starch is a major component of the human diet, providing a significant source of dietary energy. Various starchy foods, including rice, potatoes, wheat, corn, and cassava, are staples in many cultures worldwide. The nutritional value of starch comes from the readily available glucose released upon digestion, providing a sustained energy source for bodily functions.
Beyond its nutritional role, starch finds extensive applications in various industries:
-
Food Industry: Starch is used as a thickener, stabilizer, and binder in a wide range of processed foods, from baked goods and sauces to confectionery and dairy products. Modified starches with altered properties are also widely used to enhance the texture and stability of food products.
-
Textile Industry: Starch is used as a sizing agent in the textile industry to improve the strength and weaving properties of fabrics.
-
Paper Industry: Starch serves as a binder and coating agent in paper manufacturing.
-
Pharmaceutical Industry: Starch is used as a tablet binder and excipient in pharmaceutical formulations.
Conclusion: Starch – A Complex and Crucial Polymer
In conclusion, starch is unequivocally a polymer of glucose, specifically α-D-glucose. However, its simple monomeric composition belies its complex structural and functional diversity. The interplay between amylose and amylopectin, their unique glycosidic linkages, and their organization within starch granules dictate the properties that make starch crucial for plant biology, human nutrition, and numerous industrial applications. Understanding the intricacies of starch structure and function provides insights into both fundamental biological processes and the technological applications of this ubiquitous polysaccharide. Further research continues to unveil new facets of starch's complexity and potential, promising continued innovation in diverse fields.
Latest Posts
Latest Posts
-
Is Template Strand 3 To 5
Mar 19, 2025
-
What Is The Function Of The Arm Of The Microscope
Mar 19, 2025
-
The Amount Of Energy In Food Is Measured In
Mar 19, 2025
-
Which Is Greater 1 3 Or 2 5
Mar 19, 2025
-
Gay Lussacs Law Real Life Example
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Starch A Polymer Of Glucose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
