Is Soure A Acid Or Base
Juapaving
Mar 21, 2025 · 6 min read
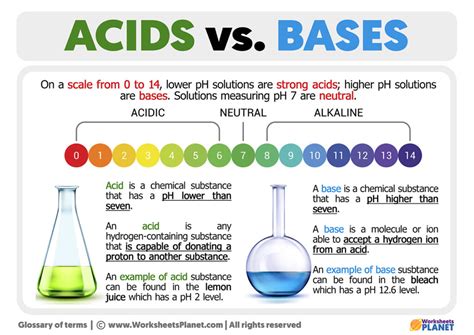
Table of Contents
Is Sour a Taste or an Acid? Understanding Acidity and its Relation to Taste
The simple answer to the question, "Is sour an acid or a base?" is: sour is a taste associated with acids. While not all acids taste sour, the sour taste we experience is almost always due to the presence of an acid. Understanding this connection requires exploring the concepts of acids, bases, and the pH scale. This article delves deep into the chemistry behind sourness and its relationship to acidity, exploring various types of acids, their properties, and their significance in our daily lives.
What are Acids and Bases?
Before we delve into the connection between sourness and acids, let's establish a clear understanding of what acids and bases are. These are fundamental concepts in chemistry, defined by their properties and behavior in chemical reactions.
Acids:
Acids are substances that donate protons (hydrogen ions, H⁺) when dissolved in water. They exhibit several characteristic properties:
- Sour Taste: This is the most readily recognizable property, although it's crucial to remember never to taste unknown substances to test for acidity.
- React with Metals: Acids react with many metals, producing hydrogen gas (H₂) and a salt. For example, hydrochloric acid (HCl) reacting with zinc (Zn) produces zinc chloride (ZnCl₂) and hydrogen gas.
- Change the Color of Indicators: Certain substances called indicators change color in the presence of acids. Litmus paper, for example, turns red in acidic solutions.
- Lower pH: Acids have a pH value less than 7 on the pH scale (explained below).
Common examples of acids include:
- Hydrochloric acid (HCl): Found in stomach acid, crucial for digestion.
- Sulfuric acid (H₂SO₄): A strong acid used in various industrial processes.
- Acetic acid (CH₃COOH): The main component of vinegar, giving it its sour taste.
- Citric acid (C₆H₈O₇): Found naturally in citrus fruits, responsible for their tartness.
Bases:
Bases are substances that accept protons (hydrogen ions, H⁺) or donate hydroxide ions (OH⁻) when dissolved in water. They have distinct properties opposite to those of acids:
- Bitter Taste: Bases typically have a bitter taste. Again, never taste an unknown substance to determine its basicity.
- Slippery Feel: Many bases feel slippery to the touch.
- Change the Color of Indicators: Bases change the color of indicators differently than acids. Litmus paper turns blue in basic solutions.
- Higher pH: Bases have a pH value greater than 7 on the pH scale.
Examples of bases include:
- Sodium hydroxide (NaOH): Also known as lye, a strong base used in various industrial and household applications.
- Potassium hydroxide (KOH): Another strong base with similar applications to sodium hydroxide.
- Ammonia (NH₃): A weak base commonly found in cleaning products.
- Calcium hydroxide (Ca(OH)₂): Also known as slaked lime, used in construction and agriculture.
The pH Scale: Measuring Acidity and Basicity
The pH scale is a logarithmic scale used to measure the acidity or basicity of a solution. It ranges from 0 to 14, with:
- pH 7: Neutral (pure water)
- pH < 7: Acidic (the lower the pH, the stronger the acid)
- pH > 7: Basic or alkaline (the higher the pH, the stronger the base)
The pH scale is crucial in many aspects of life, from understanding soil conditions in agriculture to monitoring the acidity of our blood in medicine. The sour taste we associate with acidic substances is directly linked to their position on this scale.
The Chemistry of Sourness: Why Acids Taste Sour
The sour taste is a sensory perception triggered by the presence of hydrogen ions (H⁺) in our food and drinks. When we consume an acidic substance, the hydrogen ions interact with taste receptors on our tongue, specifically those responsible for detecting sourness. The intensity of the sour taste correlates with the concentration of hydrogen ions – higher concentration means a more intensely sour taste.
Different Acids, Different Sourness:
While all acids produce hydrogen ions and contribute to sourness, the specific type of acid and its concentration significantly influence the perceived taste. For instance:
- Citric acid in lemons produces a bright, sharp sourness.
- Acetic acid in vinegar provides a milder, more vinegary sourness.
- Tartaric acid in grapes offers a unique tartness.
- Malic acid in apples gives a slightly less intense sourness compared to citrus fruits.
The differences are not simply about the intensity of the sourness but also about other sensory attributes that contribute to the overall taste experience. These attributes often involve the interplay of other compounds present in the food or beverage along with the acid.
Sourness Beyond Acids: Other Contributing Factors
While acids are the primary cause of sourness, it's important to note that other factors can influence the perceived sourness of a food or drink:
- Temperature: Cold temperatures can enhance the perception of sourness, while warm temperatures can reduce it.
- Other Taste Sensations: Sweetness, saltiness, bitterness, and umami can interact with sourness, altering the overall taste perception. A balance of sweet and sour is a popular flavor combination.
- Texture: The texture of food also plays a role. A smoother texture might amplify the sourness, while a rougher texture might mask it somewhat.
- Concentration: The concentration of the acid is a crucial factor. A highly concentrated acid will taste intensely sour, while a diluted one will taste less so.
Acids in Everyday Life: A Wide Range of Applications
Acids are ubiquitous in our daily lives, playing vital roles in various aspects:
- Food and Beverages: As discussed, acids contribute significantly to the flavors of many foods and drinks, from citrus fruits to vinegar and soft drinks.
- Digestion: Hydrochloric acid in the stomach is essential for breaking down food and killing harmful bacteria.
- Industrial Processes: Strong acids like sulfuric acid and nitric acid are used in various industrial processes, including the production of fertilizers, plastics, and detergents.
- Pharmaceuticals: Many pharmaceuticals utilize acids in their formulations.
- Cosmetics and Personal Care: Some skincare products incorporate acids for exfoliation and other purposes.
Safety Precautions When Handling Acids
It is crucial to emphasize the importance of safety when handling acids, especially strong acids. Always wear appropriate safety gear, including gloves, goggles, and lab coats. Never mix acids with other chemicals without proper knowledge and safety procedures. Strong acids can cause severe burns and damage to skin and eyes upon contact.
Conclusion: Sourness is Primarily an Acidic Phenomenon
In summary, the sour taste is overwhelmingly associated with the presence of acids and their hydrogen ion concentration. While other factors can influence the overall sensory experience, the fundamental cause of sourness lies in the interaction of hydrogen ions with our taste receptors. Understanding the chemistry of acids and bases is crucial for comprehending this fundamental aspect of taste perception and the many roles acids play in our lives. Remember always to handle acids with caution and respect their potential hazards. This comprehensive explanation provides a detailed understanding of the connection between sourness and acidity, encompassing the chemical principles and their practical implications. Further research into specific acids and their properties can deepen this knowledge even further.
Latest Posts
Latest Posts
-
Is Ice Cream Melting A Physical Change
Mar 27, 2025
-
Why Does Electronegativity Increase From Left To Right
Mar 27, 2025
-
What Is The Lcm Of 25 And 35
Mar 27, 2025
-
What Are All The Factors Of 11
Mar 27, 2025
-
What Is The Factors Of 25
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Is Soure A Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
