Single Replacement Reaction Examples Real Life
Juapaving
Mar 18, 2025 · 5 min read
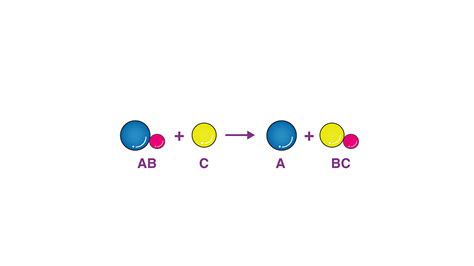
Table of Contents
Single Replacement Reaction Examples in Real Life: A Comprehensive Guide
Single replacement reactions, also known as single displacement reactions, are a fundamental type of chemical reaction where one element replaces another element in a compound. These reactions are ubiquitous in our daily lives, often unnoticed but playing crucial roles in various processes. Understanding these reactions is key to appreciating the chemistry behind many everyday phenomena and industrial processes. This comprehensive guide explores numerous real-life examples, categorized for clarity and better understanding.
Understanding Single Replacement Reactions
Before delving into the real-world applications, let's briefly revisit the core principles. A single replacement reaction follows a general pattern:
A + BC → AC + B
Where:
- A is a more reactive element.
- BC is a compound.
- AC is a new compound formed.
- B is the displaced element.
The reaction occurs only if element A is more reactive than element B. Reactivity is determined by the activity series of metals (and a similar series for non-metals, though less commonly used). The activity series ranks elements based on their tendency to lose electrons (oxidation) – a more reactive element readily loses electrons, displacing a less reactive one.
Real-Life Examples of Single Replacement Reactions
The applications of single replacement reactions are diverse and far-reaching, influencing everything from the corrosion of metals to the production of essential chemicals. Let's examine some key examples across various contexts:
1. Corrosion and Rusting: A Common Single Replacement Reaction
Rusting, the deterioration of iron into iron oxide (rust), is a classic example. Iron reacts with oxygen in the presence of water or moisture:
4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
While not strictly a simple single replacement in the A + BC format, it involves iron replacing hydrogen in water molecules and oxidizing to form iron hydroxide. This eventually converts to iron oxides (rust). The presence of an electrolyte like salt accelerates this process. This is why saltwater environments significantly increase the rate of corrosion. Similar reactions occur with other metals, albeit at varying rates.
2. Extraction of Metals: Harnessing Reactivity
The extraction of metals from their ores often involves single replacement reactions. For instance, obtaining metals like copper or silver from their ores might involve reacting the ore with a more reactive metal like iron. This process is used in hydrometallurgy. For example:
Cu²⁺(aq) + Fe(s) → Cu(s) + Fe²⁺(aq)
In this reaction, iron, being more reactive than copper, displaces copper ions from a solution, resulting in solid copper metal. This principle is applied in various metallurgical processes to refine and extract valuable metals.
3. Production of Hydrogen Gas: A Valuable Resource
Producing hydrogen gas (H₂) for various applications often relies on single replacement reactions. One common method is reacting a reactive metal like zinc or magnesium with an acid:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Here, zinc replaces hydrogen in hydrochloric acid, producing hydrogen gas and zinc chloride. This reaction is widely used in laboratory settings to generate hydrogen for experiments. Similar reactions occur with other acids and reactive metals.
4. Galvanization: Protecting Iron from Corrosion
Galvanization is a crucial process in preventing iron from rusting. It involves coating iron with a more reactive metal like zinc. If the zinc coating gets scratched, it reacts with oxygen and moisture instead of the iron underneath, thus protecting it from corrosion:
Zn(s) + O₂(g) + H₂O(l) → Zn(OH)₂(s)
This sacrificial protection is a clear application of the principles of single replacement reactions, where zinc actively prevents the oxidation of iron.
5. Photographic Development: A Chemical Reaction in Imaging
Developing photographic film involves a series of chemical reactions, some of which are single replacement reactions. Silver halide crystals in the film are exposed to light, and during development, a reducing agent reacts with the exposed silver halide, reducing the silver ions (Ag⁺) to metallic silver (Ag), forming a visible image:
AgBr(s) + reducing agent → Ag(s) + Br⁻(aq) + products
This reduction process is a form of single replacement where silver ions are replaced by the reducing agent.
6. Batteries: Powering Devices through Redox Reactions
Batteries operate based on redox reactions, where oxidation and reduction occur simultaneously. Many battery types utilize single replacement reactions as a fundamental part of their electrochemical processes. For example, in a simple zinc-carbon battery, zinc undergoes oxidation, while manganese dioxide (MnO₂) undergoes reduction. The movement of electrons during this process generates an electric current.
Zn(s) → Zn²⁺(aq) + 2e⁻ (Oxidation)
MnO₂(s) + H₂O(l) + e⁻ → MnO(OH)(s) + OH⁻(aq) (Reduction)
7. Water Treatment: Removing Impurities
Water treatment processes may sometimes utilize single replacement reactions to remove undesirable ions or impurities. For example, certain metal ions in water can be precipitated out by reacting them with a more reactive metal or by introducing a suitable reagent that displaces them.
8. Dental Fillings: Preventing Tooth Decay
The process of amalgam fillings in dentistry, where mercury reacts with other metals like silver, tin, and copper, utilizes a single replacement-type interaction. The precise reactions are complex but involve the displacement and redistribution of metal ions to form an amalgam alloy that fills cavities.
9. Chemical Synthesis: Creating New Compounds
Single replacement reactions are often employed in chemical synthesis to produce specific compounds. For example, a more reactive halide can displace a less reactive one in a compound, such as in the reaction between sodium iodide and chlorine gas:
2NaI(aq) + Cl₂(g) → 2NaCl(aq) + I₂(s)
Here, chlorine replaces iodine in sodium iodide. This principle finds numerous applications in organic and inorganic chemistry.
10. Industrial Processes: Large-Scale Applications
Many industrial processes rely on single replacement reactions for various purposes. These range from the production of various metals to the synthesis of chemicals used in manufacturing plastics, pharmaceuticals, and other materials. The scale of these operations demonstrates the significance of this fundamental reaction type.
Conclusion: The Pervasiveness of Single Replacement Reactions
Single replacement reactions are not merely textbook concepts; they are fundamental chemical processes woven into the fabric of our daily lives. From the rusting of a nail to the power generated by a battery, these reactions are constantly at play. Understanding the principles behind these reactions allows us to appreciate the chemistry that shapes our world, influencing various aspects of technology, materials science, and environmental processes. This knowledge is crucial for developing new materials, improving existing technologies, and addressing environmental challenges. Further exploration into the specific reaction mechanisms and applications in various fields can provide a deeper appreciation for the importance of single replacement reactions.
Latest Posts
Latest Posts
-
The Higher The Frequency Of A Sound Wave
Mar 18, 2025
-
Advantages Of Ac Current Over Dc
Mar 18, 2025
-
The Post Office Of The Cell Is The
Mar 18, 2025
-
The Distance Between Adjacent Wave Crests Is Called
Mar 18, 2025
-
What Energy Transformation Occurs In A Generator
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Single Replacement Reaction Examples Real Life . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
