Is Potassium A Metal Nonmetal Or Metalloid
Juapaving
Mar 26, 2025 · 5 min read
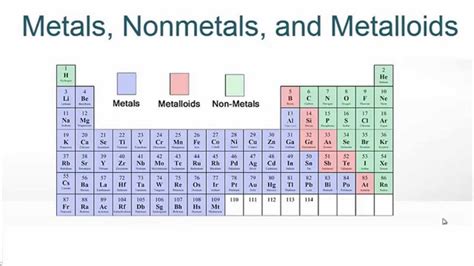
Table of Contents
Is Potassium a Metal, Nonmetal, or Metalloid? A Deep Dive into Alkali Metals
Potassium, a vital element for all living organisms, holds a prominent place in the periodic table. But where exactly does it fit? Is it a metal, a nonmetal, or a metalloid? The answer, definitively, is metal. However, understanding why potassium is classified as a metal requires delving into its physical and chemical properties, its position within the periodic table, and its unique behavior compared to nonmetals and metalloids. This comprehensive article will explore these aspects in detail, providing a thorough understanding of potassium's metallic nature.
Understanding the Classification of Elements
Before diving into the specifics of potassium, let's establish a fundamental understanding of how elements are classified. The periodic table organizes elements based on their atomic structure and properties, broadly categorizing them into metals, nonmetals, and metalloids. These categories aren't rigidly defined; there's a spectrum of properties, and some elements exhibit characteristics of multiple groups.
Metals: The Characteristics of Conductivity and Malleability
Metals, which constitute the vast majority of elements, are typically characterized by:
- Excellent electrical conductivity: They readily conduct electricity due to the presence of freely moving electrons in their outer shells. This explains why metals are used extensively in electrical wiring and circuitry.
- High thermal conductivity: They efficiently transfer heat, making them suitable for applications like cookware and heat sinks.
- Malleability and ductility: They can be easily shaped (hammered into sheets) and drawn into wires without breaking, reflecting their flexible atomic structure.
- Metallic luster: They have a characteristic shiny appearance due to the interaction of light with their delocalized electrons.
- High tensile strength: They can withstand significant pulling forces before breaking, lending themselves to structural applications.
- Low ionization energy: They readily lose electrons to form positive ions, a property crucial for their chemical reactivity.
Nonmetals: The Opposite End of the Spectrum
In contrast, nonmetals typically exhibit:
- Poor electrical and thermal conductivity: They generally don't conduct electricity or heat well, often acting as insulators.
- Brittleness: They are often fragile and break easily when subjected to stress. They lack the malleability and ductility of metals.
- Dull appearance: They lack the shiny luster characteristic of metals.
- High electronegativity: They tend to attract electrons in chemical bonds, often forming negative ions.
- High ionization energy: They strongly resist losing electrons.
Metalloids: Bridging the Gap
Metalloids occupy a fascinating middle ground, possessing properties of both metals and nonmetals. Their behavior is often context-dependent, exhibiting metallic properties under certain conditions and nonmetallic properties under others. Examples include silicon and arsenic. These elements often have semi-conductivity, meaning their electrical conductivity falls somewhere between that of metals and nonmetals. This makes them crucial in the semiconductor industry.
Potassium: A Definitive Metal
Now, let's focus on potassium (K), an element residing in Group 1 of the periodic table, also known as the alkali metals. The properties of potassium unequivocally place it in the metal category.
Potassium's Metallic Properties
- Electrical Conductivity: Potassium is an excellent conductor of electricity, like most metals. The ease with which its single valence electron moves contributes to this high conductivity.
- Thermal Conductivity: It effectively transfers heat, although not as efficiently as some other metals.
- Malleability and Ductility: Although softer than many other metals, potassium is malleable and ductile, capable of being shaped and drawn into wires.
- Metallic Luster: When freshly cut, potassium displays a silvery-white metallic luster, quickly tarnishing in air due to its high reactivity.
- Low Ionization Energy: Potassium readily loses its single valence electron to form a K⁺ ion, a defining characteristic of its reactivity and its role in chemical compounds.
- Low Density: Potassium is a relatively light metal, less dense than water. This low density is a significant property distinguishing it from many other metals.
- High Reactivity: Potassium is extremely reactive, especially with water and oxygen. This reactivity stems from its low ionization energy and its eagerness to lose its valence electron. This high reactivity necessitates careful handling and storage under inert conditions.
Why Potassium Isn't a Nonmetal or Metalloid
Potassium's properties starkly contrast with those of nonmetals. It doesn't exhibit the brittleness, poor conductivity, or high electronegativity associated with nonmetals. Similarly, it doesn't show the semiconducting behavior or variable properties that define metalloids. Its consistent display of metallic characteristics firmly places it in the metal category.
Potassium's Role in Biological Systems and Beyond
Potassium's importance extends far beyond its classification. It's an essential element for all living organisms, playing a crucial role in numerous biological processes:
- Nerve Impulse Transmission: Potassium ions are vital for the transmission of nerve impulses, enabling communication between cells. The movement of potassium ions across cell membranes generates the electrical signals that underpin the nervous system's function.
- Muscle Contraction: Potassium ions are integral to muscle contraction, contributing to the coordinated movements of muscles throughout the body. Disruptions in potassium levels can significantly impact muscle function.
- Maintaining Osmotic Balance: Potassium helps maintain the proper balance of fluids within and outside cells, ensuring cellular health and function.
- Enzyme Activation: Some enzymes require potassium ions for their proper functioning, highlighting the element's role in various metabolic pathways.
- Plant Growth: Potassium is a major macronutrient for plant growth, contributing to robust stems, strong roots, and increased yields. Potassium deficiency in plants results in stunted growth and decreased productivity.
Beyond its biological importance, potassium finds applications in various industrial processes:
- Photoelectric Cells: Potassium's photoelectric effect, where it emits electrons when exposed to light, finds use in specialized photoelectric cells.
- Production of Potassium Compounds: Potassium is used in the production of various potassium compounds, such as potassium hydroxide (KOH), used in soap manufacturing, and potassium carbonate (K₂CO₃), employed in glass production.
- Fertilizers: Potassium compounds are crucial components of many fertilizers, supplying plants with the potassium they need for healthy growth.
Conclusion: Potassium's Metallic Identity is Undisputed
The overwhelming evidence presented demonstrates that potassium is definitively a metal. Its electrical conductivity, thermal conductivity, malleability, ductility, metallic luster, and low ionization energy all align perfectly with the characteristics defining metals. Its high reactivity, while significant, doesn't negate its metallic properties. Its biological importance and industrial applications further underscore its significance in the world around us. Therefore, there's no ambiguity: potassium belongs firmly and unequivocally within the metal classification of the periodic table. Understanding its metallic nature is essential for appreciating its role in both the natural world and human applications.
Latest Posts
Latest Posts
-
Five Letter Word Starts With Vi
Mar 29, 2025
-
Why Is Air A Homogeneous Mixture
Mar 29, 2025
-
How To Simplify Square Root Of 80
Mar 29, 2025
-
How Do You Separate Oil And Vinegar
Mar 29, 2025
-
Is 2 Cm The Same As 1 Inch
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is Potassium A Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
